Introduction
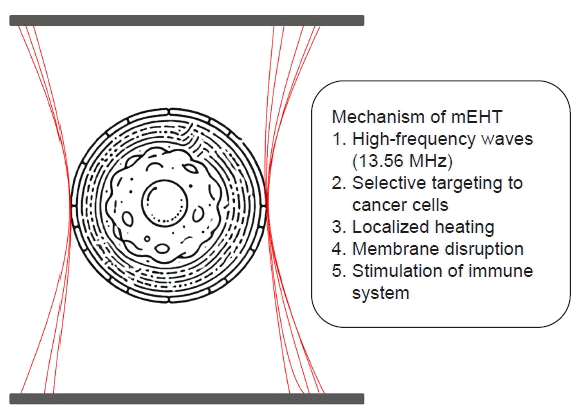
mEHT mechanism
mEHT in combination with radiation therapy
mEHT in combination with chemotherapy
Safety of mEHT
Previously published studies on hyperthermia
Table 1.
| Author | Article type | Cancer type | Intervention | Total patients | Endpoints | Oncologic outcomes | Survival outcomes | QoL outcomes | Side effects |
|---|---|---|---|---|---|---|---|---|---|
| Datta et al. (2016) [36] | Meta-analysis | Breast | RT vs. HT+RT | 627 | CR | Improved CR with HT | - | - | Minimal acute and late morbidities |
| Hu et al. (2017) [39] | Meta-analysis | Esophagus | HT+CCRT vs. CCRT or RT alone | 1,519 | OS | HT+CCRT vs. CCRT: | HT+CCRT vs. CCRT: | - | HT+CCRT vs. CCRT: |
| Long-term effects (LR and DM rate) | Improved CR (OR, 2.00; p<0.00001) and TER (OR, 3.47; p<0.00001) | Improved 1-, 3-, 5-, and 7-yr OS (OR 1.79, 1.91, 9.99, 9.49; p<0.05) | Less GI toxicities with HT (OR, 0.43; p<0.00001) | ||||||
| Short-term effects (CR and TER) | No difference in long-term effects | HT+CCRT vs. RT alone: | HT+CCRT vs. RT alone: | ||||||
| HT+CCRT vs. RT alone: | Improved 1-, 2-, 3-, and 5-yr OS (OR 3.20, 2.09, 2.43, 3.47, p<0.05) | No statistical difference in toxicities | |||||||
| Higher CR (OR, 2.12; p=0.003) and TER (OR, 4.8; p=0.002) | |||||||||
| Lower LR (OR, 0.39; p=0.0001) and DM (OR, 0.46; p=0.003) | |||||||||
| Van der Horst et al. (2017) [20] | Systematic review | Pancreas | HT+RT or CT or CCRT | 395 | Overall response rate | Improved overall response rate (43.9% vs. 35.3%) | Improved OS (11.7 mo vs. 5.6 mo) | - | - |
| OS | |||||||||
| De Haas-Kock et al. (2009) [46] | Systematic review | Rectum | HT+RT vs. RT alone | 520 | Pathologic | Higher CR with HT (RR, 2.81; p=0.01) | Improved 2-yr OS (HR, 2.06; p=0.001) but, disappeared in 3-, 4-, 5-yr OS | - | No difference in acute toxicity |
| CR | |||||||||
| OS | |||||||||
| Toxicity | |||||||||
| Veltsista et al. (2023) [48] | Systematic review | Soft tissue sarcoma | HT+CT or HT+RT | 786/618 | - | Increased RT effectiveness with HT | - | - | No significant toxicity of HT |
| Lutgens et al. (2010) [50] | Systemic Review | Uterine cervix | HT+RT vs. RT alone | 487 | CR | Improved CR (RR 0.56; p<0.001) and LR rate (HR 0.48; p<0.001) | Better OS (HR, 0.67; p=0.05) | - | No difference in acute or late grade 3–4 toxicity |
| LR | |||||||||
| OS | |||||||||
| Grade 3 to 4 acute and late toxicity | |||||||||
| Datta et al. (2016) [51] | Meta-analysis | Uterine cervix | HT+RT +/− CT vs. RT+/−CT | 1,160 | CR | Higher CR (+22.1%, p<0.001) and LC (+23.1%, p<0.001) with HT | No significant OS benefit | - | No difference in acute or late toxicities |
| LC | |||||||||
| OS | |||||||||
| Acute and late grade 3/4 toxicity | |||||||||
| Yea et al. (2021) [53] | Meta-analysis | Uterine cervix | HT+CCRT vs. CCRT | 536 | 5-yr OS | No LRFS benefit | Better 5-yr OS (HR, 0.67; p=0.03) | - | No difference in acute and late toxicity |
| LRFS | |||||||||
| Acute and late toxicity |
QoL, quality of life; RT, radiotherapy; HT, hyperthermia; CR, complete response; CCRT, concurrent chemoradiotherapy; OS, overall survival; LR, local recurrence; DM, distant metastasis; TER, total effective rate; OR, odds ratio; GI, gastrointestinal; CT, chemotherapy; RR, relative risk; HR, hazard ratio; LC, local control; LRFS, local relapse-free survival.
Table 2.
| Author | Article type | Cancer type | Intervention | Total patients | Endpoints | Oncologic outcomes | Survival outcomes | QoL outcomes | Side effects |
|---|---|---|---|---|---|---|---|---|---|
| Loboda et al. (2020) [37] | Phase II RCT | Breast | NACT+HT vs. NACT | 200 | Tumor response | Better tumor size reduction with HT | Higher 10-yr OS (p=0.009) | - | - |
| 10-yr OS | Objective response rate increased by 15.9% (p=0.034) | ||||||||
| Klimanov et al. (2018) [38] | Phase II RCT | Breast cancer with liver metastases | HT+CT vs. CT alone | 103 | Tumor response | Higher PR and SD | - | Improved QoL | - |
| QoL | |||||||||
| Kang et al. (2013) [40] | Phase II RCT | Head and neck | HT+CCRT vs. CCRT | 154 | CR | Higher 3-mo CR (81.6% vs. 62.8%; p<0.05) and 5-yr LC rate (96.1% vs. 76.9%) | Better 5-yr DFS (51.3% vs. 20.5%) and 3-yr (85.5% vs. 61.5%), 5-yr OS (68.4% vs. 50.0%) | - | No statistical difference in toxicity |
| DFS | |||||||||
| OS | |||||||||
| Zhao et al. (2014) [41] | Phase II RCT | Head and neck | HT+CCRT vs. CCRT | 83 | OS | - | Higher 3-yr OS (73% vs. 53.5%, p=0.048) and PFS (61 mo vs. 38 mo, p=0.048) | Better QoL with HT | - |
| DFS | |||||||||
| QoL | |||||||||
| Ren et al. (2021) [42] | Phase II RCT | Head and neck | HT+induction CT vs. induction CT alone | 120 | Clinical response rate of induction CT | Higher clinical response rates (65.45% vs. 40.00%, p=0.0088) | Improved DFS (HR, 0.57; p=0.035) not OS | - | No difference in adverse events |
| OS | |||||||||
| DFS | |||||||||
| Toxicity | |||||||||
| Dong et al. (2016) [16] | Phase II RCT | Liver | HT+RT vs. RT alone | 80 | Liver function | Improved liver function and TER (60.0% vs. 47.5%, p<0.001) | Lower 1-yr recurrence (27.5% vs. 40.0%, p<0.001) and mortality rates (12.5% vs. 20.0%, p<0.001) | - | - |
| TER (CR, PR, or SD) | |||||||||
| Recurrence and mortality rate | |||||||||
| Mitsumori et al. (2007) [43] | Phase II RCT | Lung | HT+RT vs. RT alone | 80 | LRR | No difference in LRR | Better LPFS (p=0.036) with HT | - | No grade 3 late toxicity |
| LPFS | No difference in OS | ||||||||
| OS | |||||||||
| Shen et al. (2011) [44] | Phase II RCT | Lung | CT+HT vs. CT alone | 80 | Response rate | No difference in response rate | No survival data reported | Improved QoL with HT (82.5% vs. 47.5%, p<0.05) | Toxicity was not statistically analyzed |
| QoL | |||||||||
| Toxicity | |||||||||
| Schulze et al. (2006) [45] | Phase II RCT | Rectum | HT+CCRT vs. CCRT | 137 | GI QoL index | - | - | No difference in QoL | - |
| Issels et al. (2018) [47] | Phase III RCT | Soft tissue sarcoma | HT+NACT vs. NACT alone | 341 | LPFS | - | Improved LPFS (HR, 0.65; p=0.002) and OS (HR, 0.73; p=0.04) | - | - |
| OS | Prolonged 5-yr (62.7% vs. 51.3%) and 10-yr (52.6% vs. 42.7%) survival rate | ||||||||
| Jones et al. (2005) [49] | Phase II RCT | Superficial skin tumor | HT+RT vs. RT alone | 108 | CR | Improved CR (OR, 2.7; p=0.02) | No OS benefit | - | - |
| LC | |||||||||
| OS | |||||||||
| Minnaar et al. (2022) [52] | Phase III RCT | Uterine cervix | HT+CCRT vs. CCRT | 210 | LC | Better 6-mo LC (p=0.003) and 2-yr (HR, 0.67; p=0.017) and 3-yr DFS (HR, 0.70; p=0.035) | No OS benefit (except for FIGO III) | Better QoL (pain reduction) with HT | No difference in toxicity |
| Toxicity | |||||||||
| QoL | |||||||||
| 2-yr OS | |||||||||
| Van der Zee et al. (2000) [54] | Phase III RCT | Uterine cervix and bladder | HT+RT vs. RT alone | 101 | CR | Bladder: | Bladder: | - | No difference in acute and late toxicities |
| LC | Improved CR (55% vs. 39%, p=0.001), not LC | No OS gain | |||||||
| OS | Uterine cervix: | Uterine cervix: | |||||||
| Improved CR (83% vs. 57%, p=0.003) | Improved 3-yr OS (51% vs. 27%, p=0.009) | ||||||||
| Chi et al. (2018) [55] | Phase III RCT | Painful bone metastases | HT+RT vs. RT alone | 108 | Pain response | Improved pain response (CR, 37.9% vs. 7.1%, p=0.006) | - | - | No grade 3 adverse events in both arms |
| Time to pain progression | Longer time to pain progression (HR, 0.178; p<0.001) | ||||||||
| Toxicity |
QoL, quality of life; RCT, randomized controlled trial; NACT, neoadjuvant chemotherapy; HT, hyperthermia; OS, overall survival; CT, chemotherapy; PR, partial response; SD, stable disease; CCRT, concurrent chemoradiotherapy; CR, complete response; DFS, disease-free survival; LC, local control; HR, hazard ratio; RT, radiotherapy; TER, total effective rate; LRR, local response rate; LPFS, local progression-free survival; GI, gastrointestinal; OR, odds ratio.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download