Abstract
The growing popularity of cosmetic procedures such as liposuction and fat grafting has been accompanied by a rise in associated side effects. Among these, fat embolism syndrome stands out as a potential complication that sometimes has critical consequences. It is important to recognize that fat embolism affects organs through distinct mechanisms from those involved with other types of embolisms. Early diagnosis is crucial. Unfortunately, no effective treatments have been established for this condition. Therefore, starting with a more detailed categorization of diagnoses, developing new treatment methods for each subtype is essential.
The first reported case of death due to fat embolism syndrome (FES) was documented by Zenker in 1862 [1]. Modern liposuction was first developed in the early 1980s [2], and subsequently, in 1983, Hunter et al. [3] reported on “Pulmonary complications following abdominal lipectomy,” which is considered the earliest report of FES associated with cosmetic surgery. Since then, numerous cases of FES related to cosmetic procedures have been documented, suggesting a significant correlation between the increasing prevalence of cosmetic surgeries and the incidence of FES.
FES is a potential complication that can arise from procedures such as liposuction, fat grafting, or even fractures, and in some cases, it can be life-threatening.
In adults, the diameter of adipocytes typically ranges from 20 to 200 μm, while the diameter of fat particles obtained through standard aspiration cannulas can exceed 1 mm. High-pressure injection, large-volume fat grafting, and liposuction present significant risks for developing FES [4].
In this article, we aim to provide a comprehensive overview of FES, with a focused review on the three most representative and critical forms of this condition.
Fat, the causative agent of FES can be classified into two types within the human body. One type is the mobile fat present in the bloodstream, and the other is the fat stored outside the blood vessels. Mobile fat exists in the form of chylomicrons bound to apolipoproteins. Stored fat accumulates as triglycerides (TG) within adipocytes. TG can be hydrolyzed into free fatty acids (FFAs) by lipase, and these FFAs can bind to albumin and be transported through the bloodstream to other tissues [5].
FFAs are considered toxic substances. They bind with calcium ions in the blood, disrupting the junctions between endothelial cells. This results in damage to the endothelial surface of capillaries, increasing vascular permeability and leading to hemorrhage and edema [6].
The progression from asymptomatic fat embolism to severe FES can be explained by two mechanisms. One is the mechanical obstruction theory, which posits that when adipose tissue is damaged, peripheral vessels are mechanically obstructed by fat emboli, leading to ischemia and severe dysfunction of distal organs [7]. This condition is known as fulminant acute FES. Rapidly progressing clinical symptoms require a significant amount of fat emboli (Fig. 1). However, FES resulting from mechanical obstruction is rare. The other theory is the chemical theory, which suggests that FFAs damage organs and cause subacute FES [6]. Subacute FES is the most common clinical type and typically occurs 12 to 48 hours post-injury (Fig. 2).
Peltier [8] was the first to explain these two theories through animal experiments. He discovered that when chemically pure triolein, a type of TG, was injected into the veins of rabbits, the LD50 (lethal dose for 50%) was relatively high at 900 mg/kg. Through gross and microscopic examination, he observed right ventricular hypertrophy and ischemic changes in the lungs, providing evidence for the mechanical obstruction theory. Additionally, he injected three types of FFAs (oleic acid, linoleic acid, and linolenic acid), finding LD50 values of 250 mg/kg, 225 mg/kg, and 200 mg/kg, respectively. These are relatively low doses. Autopsy results showed no right ventricular hypertrophy, but there was diffuse hemorrhage in the lungs, suggesting the biochemical toxicity of FFAs. These findings laid the foundation for our understanding of the pathogenesis of FES.
In FES, the fat emboli entering the circulatory system are primarily in the form of adipocytes and/or liquid fat in the form of TG from destroyed adipocytes. Therefore, the two main theories of FES (mechanical obstruction and biochemical mechanisms) are likely interconnected and do not operate independently [9]. That is, if the amount of fat entering the bloodstream is insufficient to cause fulminant FES, the TG fat emboli are hydrolyzed into FFAs. This conversion leads to a transition from mechanical obstruction to a biochemical phase, resulting in subacute FES [9-11].
There is a common misconception that FES only occurs due to the liquid component of fat. However, research indicates that not only liquid fat, but also fat cells can act as embolic material [12]. Fat cells can cause occlusion on their own, and they can also trigger inflammatory responses that promote thrombus formation, leading to mechanical occlusion [13,14]. Additionally, based on the results of the rabbit experiment with a 700 mg/kg intravenous injection of triolein, liquid TG can also act as emboli, initially presenting macroscopic pathological symptoms like occlusion and immediate organ ischemia, and over time, microscopic pathological changes resulting from chemical reactions lead to bleeding and typical symptoms of FES [9].
Unlike other embolisms, a significant characteristic of FES is the transition from ischemic symptoms to hemorrhagic symptoms as time progresses [9-11]. The precise mechanism by which FES induces bleeding remains unclear. One theory suggests that the lipoprotein lipase acts on intravascular fat, generating FFAs that induce chemokine-derived cell infiltration, thereby damaging cell-capillary walls [15]. Another theory posits a response of the lung to release lipase. This enzyme hydrolyzes fat particles, releasing FFAs and glycerol into the circulation, significantly increasing capillary permeability. This can disrupt cellular structure and lead to edema and bleeding [16,17].
One mechanism underlying iatrogenic FES that we wish to elucidate in this article is the injection of fat into the bloodstream. Therefore, the quantity and pressure of injection are crucial. The volume required to generate this reflux does not need to be high. Injecting 3 mL of saline solution containing isotopes rapidly into the radial artery was sufficient to be detected at the carotid-subclavian junction [17]. Khan et al. [18] demonstrated that even injecting less than 0.1 mL of saline solution into the supratrochlear artery of the forehead was sufficient to reach bifurcation at the orbital apex. Moreover, with sufficient pressure to overcome the blood pressure of punctured small arteries, retrograde flow can be generated, potentially affecting other distal organs [19].
Understanding these basic mechanisms is crucial for identifying specific complications associated with FES. Among them, the most severe and clinically significant manifestations are pulmonary fat embolism (PFE), cerebral fat embolism (CFE), and vision loss due to fat embolism. If these conditions are not promptly recognized and treated, they can be life-threatening or lead to serious sequelae.
PFE stands as one of the most severe complications associated with liposuction and fat grafting. During surgery, damage to adipose tissue and perforation of small blood vessels can lead to the formation of fat particles. These particles enter the venous system, causing pulmonary injury [20]. Upon manifestation of symptoms such as altered mental status, neurological deficits, or skin rash, suspicion of PFE should be promptly raised and confirmed through diagnostic testing.
The incidence of CFE has significantly increased since 2010, with the majority of cases occurring in young women of East Asian descent [21]. This trend may be attributed to the preference for facial contouring procedures in this region. One possible mechanism of CFE involves inadvertent puncture of small facial veins or arteries during facial fat grafting procedures, causing fat particles to reflux under excessive pressure into larger vessels in the vicinity, eventually flowing downstream to more distant vasculature [22]. When considering venous entry, one might wonder CFE occurs. Without a patent foramen ovale, emboli usually get trapped in the lungs, causing PFE, and it is hard to see how they could reach the brain. However, small flexible fat globules can pass through the lung capillaries, so CFE can happen even without a patent foramen ovale [23]. When fat emboli enter the cerebral circulation via the venous system, neurological deficits may manifest late, and signs of PFE such as dyspnea and tachycardia may be present. When fat embolism occurs via arterial routes, fat particles can inadvertently enter superficial arteries such as the angular artery, facial artery, or superficial temporal artery, eventually reaching the cerebral circulation due to hemodynamics [24]. Even small amounts of fat are sufficient to induce reflux. The smallest reported amount of fat causing cerebral embolism is 0.5 mL [25].
Similarly, inadvertent injection of fat into arteries during facial fat grafting procedures can lead to fat embolism traveling through the bloodstream, resulting in ophthalmic artery and central retinal artery occlusion. In such cases, all patients have experienced sudden blindness [26]. Complications related to fat injection in areas such as the forehead, temples, and glabella are more frequent [27]. Considering the vascular anatomical structure of these areas, injected fat can retrogradely reach ophthalmic artery through the internal carotid artery, or it can access the ophthalmic artery through angiosomes directly connected to the distal branches of the external carotid artery [16].
FES is primarily a diagnosis of exclusion. Various criteria aiding diagnosis have been proposed by Gurd and Wilson [28], Schonfeld [29], and Lindeque [30] (Table 1). According to Gurd and Wilson's diagnostic criteria, the presence of at least two major signs or one major sign along with four minor signs is required for a diagnosis of FES. Using Schonfeld's criteria, a score of 5 or more is sufficient for diagnosis, while Lindeque's criteria allow for a diagnosis if even one criterion is met. However, from a broader perspective, respiratory dysfunction, neurological symptoms, and petechial rash are the primary indicators of FES. Petechial rash typically appears within 3 days of symptom onset and is considered the only pathognomonic feature of FES. Nevertheless, the absence of petechial rash should not exclude the diagnosis of FES.
The symptoms of PFE are similar to many surgery-related complications such as adult respiratory distress syndrome (ARDS), pulmonary edema, aspiration pneumonia, drug allergy, transfusion-related acute lung injury, and other type of pulmonary embolisms. It is challenging to immediately distinguish PFE from these complications [31]. Typical symptoms of PFE include dyspnea, hypotension, tachycardia, hypoxemia, and altered mental status. Among patients, 23% initially presented with cardiac arrest. The range of symptom onset was from 0 to 13 days post-surgery, with the majority (69%) experiencing symptoms within 24 hours postoperatively. Laboratory tests showed a decrease in the PaO2/FiO2 ratio (PaO2/FiO2 <200) in all patients. Additionally, leukocytosis, anemia, thrombocytopenia, and elevated D-dimer levels were commonly observed [32]. The most effective strategy for rapid and accurate diagnosis is a spiral chest computed tomography (CT) scan, which typically shows diffuse mixed ground-glass and consolidation opacities in both lungs. Bronchoalveolar lavage fluid often contains bloody secretions, and microscopic examination typically reveals lipid-laden macrophages [33]. Pulmonary angiography may demonstrate multiple irregular defects in the peripheral pulmonary artery system, indicating microembolism [34-36]. Most reported plain chest X-ray (CXR) findings indicated bilateral lung opacities similar to those seen in pulmonary edema or ARDS. However, 14.3% of reported CXR findings were negative [29]. During the acute phase, echocardiography showed abnormalities such as global hypokinesis, right ventricular dilation, and signs of pulmonary hypertension in 64% of cases [37,38].
The most common symptoms of CFE are altered consciousness, hemiplegia, aphasia, and vision loss. Distinguishing fat embolism from other types of cerebral infarction on non-contrast CT is challenging. Multiple small, non-confluent hyperintense lesions in the periventricular, subcortical, and deep white matter regions, known as the “starfield pattern,” are diagnostic features of CFE on T2-weighted magnetic resource imaging (MRI) images [39,40]. The severity of CFE is graded by an MRI-based system proposed by Takahashi et al. (Table 2) [41]. Additionally, diffusion-weighted imaging (DWI) is one of the useful tools for identifying infarction which displays sporadic hyperintense lesions, resembling “a starry sky.” However, Lee et al. [42] considered magnetic resonance spectroscopy more useful than DWI for identifying fat emboli. It is notable that, although the primary concern is embolism, there are also numerous reports of associated hemorrhage [10,43], which is thought to be clinically manifested through biochemical mechanisms induced by FFAs [9]. Microhemorrhages in the “nut kernel pattern” on susceptibility-weighted imaging or T2* MRI, along with the starfield pattern and corpus callosum diffusion restriction on DWI, appear to be the most critical imaging markers of CFE and can assist in differential diagnosis in clinically ambiguous cases [10].
All patients experienced sudden blindness, ophthalmoplegia, and reduced or absent direct pupillary light reflex during the procedure. Occasionally, eye pain, ptosis, and strabismus were also observed. Some patients developed skin necrosis at the injection site, and imaging revealed cerebral microinfarctions. After conservative treatment and intra-arterial thrombolysis, visual symptoms improved with notable improvement in ptosis and ocular motility. A minority of patients experienced an improvement in vision, and significantly decreased the extent of skin necrosis [26].
All patients require conservative treatment based on their symptoms. This includes oxygen therapy, monitoring of vital signs, intravenous fluid therapy, glucocorticoid pulse anti-inflammatory treatment, reduction of intracranial or intraocular pressure, vasodilators to improve circulation, nutritional and neuroprotective therapy. If infection is evident, anti-inflammatory and antibiotic treatments are necessary. While hyaluronidase is known to be effective for vascular embolism caused by hyaluronic acid injections, there is no evidence to support its efficacy in treating FES [44]. Patients with skin necrosis should undergo debridement of the affected area and treatment with tissue growth factors. For patients with cerebral infarction, free radical scavengers may be administered to protect brain cells [26]. Administration of albumin appears to be beneficial, likely due to its ability to bind FFAs [45,46].
Primary treatment for patients with PFE includes supplemental oxygen, ventilatory support, and supportive therapy with vasodilators. Antibiotics are used to prevent or treat wound infections and pneumonia [32]. Severe PFE patients have survived after receiving extracorporeal membrane oxygenation (ECMO) [47]. ECMO is associated with better prognosis in PFE [48,49]. Acute cardiopulmonary decompensation in FES is a critical condition requiring aggressive life support measures, including ECMO. The indication for ECMO should be understood as acute cardiopulmonary collapse due to massive non-thrombotic pulmonary embolism, which is not adequately managed by general resuscitative measures alone. ECMO is typically considered in situations where conventional therapies fail and there is severe compromise of cardiac or respiratory function, generally at a mortality risk of over 50%, and is used almost universally at a mortality risk of 80% [48]. In extreme PFE cases, patients may undergo intravenous streptokinase infusion, pulmonary artery catheter embolectomy, or lobectomy [34]. For immobilized patients, thromboembolism prophylaxis should be initiated [50]. Among reported severe PFE cases, 76% required mechanical ventilation, 38% experienced cardiac arrest, and 34% resulted in death. Early symptom onset correlated with a more severe clinical course [32].
Treatment options for CFE resulting from large vessel occlusion are limited and often ineffective. Early reperfusion is critical. Typically, intra-arterial mechanical thrombectomy or thrombolysis is preferred, while intravenous thrombolysis is less effective [24]. However, in cases of complete occlusion where mechanical removal is unsuccessful, urokinase is not recommended due to the risk of inducing secondary intracerebral hemorrhage. Studies on CFE caused by fat injection have shown that intravenous thrombolysis is not only ineffective for non-thrombotic embolism but can also exacerbate the patient's condition by causing drug-induced intracerebral hemorrhage [26]. Timely recognition and intervention are crucial for successful outcomes. Zhou and Cai [51] demonstrated significant neurological improvement in a group treated with stent placement and mechanical fat aspiration. Despite attempts to use steroids to suppress the inflammatory cascade, the results were unsatisfactory. Symptomatic treatment, including intracranial pressure reduction, anti-infective therapy, nutritional support, and neuroprotection is essential. Despite these interventions, the prognosis remains poor [24]. The mortality rate was 20.6%. Early symptom onset confirmed by MRI and higher severity on the Takahashi grading scale were better predictors of mortality, whereas age was not [52].
Irreversible damage to retinal neurons caused by ischemia occurs within 90 minutes to 4 hours [53]. Most studies have shown that faster treatment leads to better visual improvement [54]. Selective angiography is necessary to confirm embolism in the retinal artery and its branches. Upon confirmation of the embolism, mechanical recanalization of the occluded vessel using a micro-guidewire and the administration of papaverine and urokinase into the ophthalmic artery shown good results [26]. Notably, when the catheter guided by the wire enters very small vessels such as the ophthalmic artery, it may cause arterial dissection or other injuries. Therefore, forced thrombectomy is not recommended [26]. While symptoms such as ptosis, ischemia of the skin and soft tissues, and strabismus improved following treatment, visual improvement was rare and often incomplete [55]. For optimal outcomes, treatment for retinal ischemia should be initiated within 90 minutes to 4 hours of symptom onset. Early intervention increases the likelihood of visual improvement. In cases of partial vascular occlusion, timely restoration of blood flow is crucial. Reperfusion therapy administered within approximately 6 hours of mechanical recanalization can also enhance visual outcomes. The severity of the patient's vascular occlusion may have a more significant impact on long-term visual outcomes [54]. Particularly, patients receiving only conservative treatment tend to experience irreversible vision loss [27].
To date, the treatment methods for FES, along with the associated outcomes and prognosis, remain severe, highlighting the importance of early intervention and prevention.
The high mortality rate and the lack of effective treatments for fat embolism emphasize the necessity for preventions. To minimize the occurrence of life-threatening complications associated with liposuction and fat grafting, the following actions are recommended. Patients should be informed that vision loss, respiratory dysfunction, and neurological impairment can occur within the first 4 days post-procedure, and immediate medical support is crucial. Therefore, patients should not be left alone on the first day after the procedure [16]. Surgeries should adhere strictly to standardized protocols, with careful monitoring of the patient's vital signs and neurological symptoms. Local anesthesia is preferred over general or intravenous anesthesia when possible. Physicians must have a thorough understanding of facial vascular anatomy to avoid inadvertent intravascular fat injection. Injection sites must be selected with meticulous care. Although grafted fat tissue tends to survive better in highly vascular areas, this increases the risk of vascular injury [24]. Ultrasound-guided injections or the use of epinephrine before surgery can reduce vascular damage [16]. Kadouch et al. [56] demonstrated the enhanced safety of temporal fat grafting using duplex-ultrasound guidance. For gluteal augmentation, fat tissue should be placed in the subcutaneous space above the deep muscle plane and gluteal fascia plane using ultrasound guidance [47,57,58]. Some authors suggest that using blunt-tipped, large-diameter cannulas instead of needles may be preferable to avoid puncturing small branch vessels [22,59,60]. Fat particles should be injected slowly at low pressure while retracting the syringe [18,61]. Applying digital pressure proximal to the vessel during injections in high-risk areas can prevent the retrograde flow of fat into the bloodstream [62]. Injecting smaller volumes than those required to reach the ophthalmic artery or ICA can reduce complications [18]. For safety, a 1 mL multi-channel syringe can be utilized, and injections should be administered very slowly, especially in high-risk areas, with the syringe withdrawn at low pressure [15,63]. Currently, no medications have been proven to prevent the occurrence of fat embolism or alleviate its symptoms.
Treatment failure in fat embolism can stem from three main reasons. Firstly, there is confusion due to the generalized diagnosis of FES without further differentiation. Fat embolism resulting from fractures is sometimes referred to as bone marrow embolism. This implies the presence of various embolic materials, including not only liquid fat but also fat cells, hematopoietic cells, and others [64]. Unlike air embolism or thrombotic or septic embolism [65,66], which occur due to a single substance or mechanism, fat embolism involves diverse embolic materials and mechanisms. In other words, the embolic material in fat embolism can be either fat cells themselves or in the form of liquid fat, and the mechanisms of occurrence can involve mechanical occlusion or chemical action. These distinctions need to be accurately identified to enable proper comparison of diagnosis, treatment, and outcomes, etc. and to facilitate the development of new treatments. Secondly, there are inherent limitations of thrombectomy materials [24]. Currently available stents are primarily designed with open or coil like closed-loop structures, which can effectively capture solid thrombi but are less efficient in capturing liquid emboli. Thirdly, very small, disseminated fat emboli can induce microcirculatory disturbances, reducing the potential benefits of embolectomy. Additionally, while hyaluronic acid fillers contain hyaluronidase as a solvent, there is no solvent in fat cells or liquid fat. Moreover, TG naturally break down to form FFAs, which can lead to secondary vascular damage and bleeding [9].
Effective treatment for FES demands clear differentiation between liquid fat and fat cell emboli. Liquid fat FES requires chemical treatments, with drugs like albumin targeting FFAs showing promise. Thrombolytics, such as urokinase, should be used cautiously, as they can exacerbate bleeding in non-thrombotic cases. Fat cell-related FES needs physical removal and often coexists with liquid fat emboli. Developing advanced devices for safely extracting emboli from delicate vessels, like the central retinal and ophthalmic arteries, is essential.
References
1. Zenker FA; Royal College of Physicians of Edinburgh. Beiträge zur normalen und pathologischen Anatomie der Lunge. G. Schönfeld; 1862.
2. Illouz YG. Body contouring by lipolysis: a 5-year experience with over 3000 cases. Plast Reconstr Surg. 1983; 72:591–7.
3. Hunter GR, Crapo RO, Broadbent TR, Woolf RM. Pulmonary complications following abdominal lipectomy. Plast Reconstr Surg. 1983; 71:809–17.

4. Lee JS, Kim JY, Jung C, Woo SJ. Iatrogenic ophthalmic artery occlusion and retinal artery occlusion. Prog Retin Eye Res 2020;78:100848.
5. Murray RK, Granner DK, Mayes PA, Rodwell VR. Harper's biochemistry. 25th ed. McGraw-Hill; 2000.
6. Lehman EP, Moore RM. Fat embolism: including experimental production without trauma. Arch Surg. 1927; 14:621–62.
8. Peltier LF. Fat embolism. III. The toxic properties of neutral fat and free fatty acids. Surgery. 1956; 40:665–70.
9. Kim HI, In SK, Yi HS, Kim HY, Kim YS. Experimentally induced fat embolism syndrome: shift from obstruction to toxic effects. Arch Aesthetic Plast Surg. 2021; 27:47–55.

10. Giyab O, Balogh B, Bogner P, Gergely O, Toth A. Microbleeds show a characteristic distribution in cerebral fat embolism. Insights Imaging. 2021; 12:42.
11. Kuo KH, Pan YJ, Lai YJ, Cheung WK, Chang FC, Jarosz J. Dynamic MR imaging patterns of cerebral fat embolism: a systematic review with illustrative cases. AJNR Am J Neuroradiol. 2014; 35:1052–7.

12. Lee HS, Park JJ, Roh HG, Lim SD. Unusual clinicopathological presentation of nontraumatic cerebral fat embolism: two-case report. Medicine (Baltimore). 2020; 99:e19574.
13. Safran T, Abi-Rafeh J, Alhalabi B, Davison PG. The potential role of corticosteroid prophylaxis for the prevention of microscopic fat embolism syndrome in gluteal augmentations. Aesthet Surg J. 2020; 40:78–89.

14. Duran H, Cardenas-Camarena L, Bayter-Marin JE, Ramos-Gallardo G, Robles-Cervantes JA. Microscopic and macroscopic fat embolism: solving the puzzle with case reports. Plast Reconstr Surg. 2018; 142:569e–577e.
15. Seixas E, Ferreira PG. Fat embolism syndrome presenting as diffuse alveolar hemorrhage: a rare (known) association. Pulmonology. 2018; 24:314–5.
16. Dhooghe NS, Maes S, Depypere B, Claes KE, Coopman R, Kubat B, et al. Fat embolism after autologous facial fat grafting. Aesthet Surg J. 2022; 42:231–8.

17. Turkmen Samdanci E, Reha Celik M, Pehlivan S, Celbis O, Turkkan D, Ozdemir Kara D, et al. Histopathological evaluation of autopsy cases with isolated pulmonary fat embolism (IPFE): is cardiopulmonary resuscitation a main cause of death in IPFE? Open Access Emerg Med. 2019; 11:121–7.
18. Khan TT, Colon-Acevedo B, Mettu P, DeLorenzi C, Woodward JA. An anatomical analysis of the supratrochlear artery: considerations in facial filler injections and preventing vision loss. Aesthet Surg J. 2017; 37:203–8.

19. Shiffman MA. Fat tissue embolism caused by autologous fat transfer. Am J Cosmet Surg. 2015; 32:247–53.

20. Cantu CA, Pavlisko EN. Liposuction-induced fat embolism syndrome: a brief review and postmortem diagnostic approach. Arch Pathol Lab Med. 2018; 142:871–5.
21. Wang K, Rong X, Dang J, Yang J, Zheng H, Hou M, et al. Severe vascular complications caused by facial autologous fat grafting: a critical review. Ann Plast Surg. 2021; 86(3S Suppl 2):S208–19.
22. Wang HC, Yu N, Wang X, Dong R, Long X, Feng X, et al. Cerebral embolism as a result of facial filler injections: a literature review. Aesthet Surg J. 2022; 42:NP162–75.

23. Byrick RJ, Mullen JB, Mazer CD, Guest CB. Transpulmonary systemic fat embolism: studies in mongrel dogs after cemented arthroplasty. Am J Respir Crit Care Med. 1994; 150(5 Pt 1):1416–22.

24. Cheng Y, Yan G, Li C, Han X, Shang J, Shang S, et al. Case report and literature review: fatal cerebral fat embolism following facial autologous fat graft. Front Neurol. 2023; 14:1180333.

25. Lee DH, Yang HN, Kim JC, Shyn KH. Sudden unilateral visual loss and brain infarction after autologous fat injection into nasolabial groove. Br J Ophthalmol. 1996; 80:1026–7.
26. Wu Q, Zhou G, Xu X, Liu B, Fu Q, Zhang J, et al. Exploring superselective intraarterial thrombolysis for autologous fat injection-induced vision loss. Aesthet Surg J. 2024; 44:NP337–46.

27. Moellhoff N, Kuhlmann C, Frank K, Kim BS, Conte F, Cotofana S, et al. Arterial embolism after facial fat grafting: a systematic literature review. Aesthetic Plast Surg. 2023; 47:2771–87.

29. Schonfeld SA, Ploysongsang Y, DiLisio R, Crissman JD, Miller E, Hammerschmidt DE, et al. Fat embolism prophylaxis with corticosteroids: a prospective study in high-risk patients. Ann Intern Med. 1983; 99:438–43.
30. Lindeque BG, Schoeman HS, Dommisse GF, Boeyens MC, Vlok AL. Fat embolism and the fat embolism syndrome: a double-blind therapeutic study. J Bone Joint Surg Br. 1987; 69:128–31.
31. He Z, Shi Z, Li C, Ni L, Sun Y, Arioli F, et al. Single-case metanalysis of fat embolism syndrome. Int J Cardiol. 2021; 345:111–7.

32. Kao YM, Chen KT, Lee KC, Hsu CC, Chien YC. Pulmonary fat embolism following liposuction and fat grafting: a review of published cases. Healthcare (Basel). 2023; 11:1391.

33. Kadar A, Shah VS, Mendoza DP, Lai PS, Aghajan Y, Piazza G, et al. Case 39-2021: a 26-year-old woman with respiratory failure and altered mental status. N Engl J Med. 2021; 385:2464–74.

34. Christman KD. Death following suction lipectomy and abdominoplasty. Plast Reconstr Surg. 1986; 78:428.

35. Laub DR Jr, Laub DR. Fat embolism syndrome after liposuction: a case report and review of the literature. Ann Plast Surg. 1990; 25:48–52.
36. Currie I, Drutz HP, Deck J, Oxorn D. Adipose tissue and lipid droplet embolism following periurethral injection of autologous fat: case report and review of the literature. Int Urogynecol J Pelvic Floor Dysfunct. 1997; 8:377–80.

37. Gleeson CM, Lucas S, Langrish CJ, Barlow RJ. Acute fatal fat tissue embolism after autologous fat transfer in a patient with lupus profundus. Dermatol Surg. 2011; 37:111–5.

38. Byeon SW, Ban TH, Rhee CK. A case of acute fulminant fat embolism syndrome after liposuction surgery. Tuberc Respir Dis (Seoul). 2015; 78:423–7.

39. Stoeger A, Daniaux M, Felber S, Stockhammer G, Aichner F, zur Nedden D. MRI findings in cerebral fat embolism. Eur Radiol. 1998; 8:1590–3.

40. Parizel PM, Demey HE, Veeckmans G, Verstreken F, Cras P, Jorens PG, et al. Early diagnosis of cerebral fat embolism syndrome by diffusion-weighted MRI (starfield pattern). Stroke. 2001; 32:2942–4.
41. Takahashi M, Suzuki R, Osakabe Y, Asai JI, Miyo T, Nagashima G, et al. Magnetic resonance imaging findings in cerebral fat embolism: correlation with clinical manifestations. J Trauma. 1999; 46:324–7.
42. Lee KM, Kim EJ, Jahng GH, Chang DI. Magnetic resonance findings in two episodes of repeated cerebral fat embolisms in a patient with autologous fat injection into the face. J Korean Neurosurg Soc. 2012; 51:312–5.

43. Algahtani HA, Shirah BH, Abdelghaffar N, Alahmari F, Alhadi W, Alqahtani SA. Cerebral fat embolism syndrome: diagnostic challenges and catastrophic outcomes: a case series. J Yeungnam Med Sci. 2023; 40:207–11.

44. Zhang LX, Lai LY, Zhou GW, Liang LM, Zhou YC, Bai XY, et al. Evaluation of intraarterial thrombolysis in treatment of cosmetic facial filler-related ophthalmic artery occlusion. Plast Reconstr Surg. 2020; 145:42e–50e.

45. Taviloglu K, Yanar H. Fat embolism syndrome. Surg Today. 2007; 37:5–8.
46. Chieng H, Saha B, Foulke L, Wu GP, Chopra A. A 24-year-old man with dyspnea and a broken left femur. Chest. 2022; 161:e225–31.

47. Wolfe EM, Weber LE, Wo LM, Samaha MJ, Mathew P, Garcia O, et al. Two cases surviving macro fat emboli complications following gluteal fat grafting. Aesthet Surg J. 2022; 42:902–6.

48. Lari A, Abdulshakoor A, Zogheib E, Assaf N, Mojallal A, Lari AR, et al. How to save a life from macroscopic fat embolism: a narrative review of treatment options. Aesthet Surg J. 2020; 40:1098–107.

49. Kim WJ, Kang JG. A case of rescuing a patient with acute cardiovascular instability from sudden and massive intraoperative pulmonary thromboembolism by extracorporeal membrane oxygenation. Kosin Med J. 2018; 33:477–82.

50. Anderson DR, Morgano GP, Bennett C, Dentali F, Francis CW, Garcia DA, et al. American Society of Hematology 2019 guidelines for management of venous thromboembolism: prevention of venous thromboembolism in surgical hospitalized patients. Blood Adv. 2019; 3:3898–944.
51. Zhou K, Cai C. The successful mechanical lipectomy treatment of cerebral fat embolism following autologous fat injection. Plast Reconstr Surg Glob Open. 2019; 7:e2091.

52. Armstrong BR, Devendra A, Pokale S, Subramani B, Rajesh Babu V, Ramesh P, et al. Can the rate of mortality and neurological recovery be predicted from the time of onset of symptoms and MRI grade in patients with cerebral fat embolism? A study of 34 patients. Bone Joint J. 2022; 104-B:142–9.
53. Mac Grory B, Lavin P, Kirshner H, Schrag M. Thrombolytic therapy for acute central retinal artery occlusion. Stroke. 2020; 51:687–95.

54. Tobalem S, Schutz JS, Chronopoulos A. Central retinal artery occlusion: rethinking retinal survival time. BMC Ophthalmol. 2018; 18:101.
55. Zhang L, Luo Z, Li J, Liu Z, Xu H, Wu M, et al. Endovascular hyaluronidase application through superselective angiography to rescue blindness caused by hyaluronic acid injection. Aesthet Surg J. 2021; 41:344–55.
56. Kadouch J, Schelke LW, Swift A. Ultrasound to improve the safety and efficacy of lipofilling of the temples. Aesthet Surg J. 2021; 41:603–12.

57. Mofid MM, Teitelbaum S, Suissa D, Ramirez-Montanana A, Astarita DC, Mendieta C, et al. Report on mortality from gluteal fat grafting: recommendations from the ASERF Task Force. Aesthet Surg J. 2017; 37:796–806.

58. Florida Department of State. 64B8ER19-1 Standard of care for office surgery [Internet]. Florida Department of State; c2019 [cited 2024 Aug 30]. https://www.flrules.org/gateway/ruleNo.asp?id=64B8ER19-1.
59. Hufschmidt K, Bronsard N, Foissac R, Baque P, Balaguer T, Chignon-Sicard B, et al. The infraorbital artery: clinical relevance in esthetic medicine and identification of danger zones of the midface. J Plast Reconstr Aesthet Surg. 2019; 72:131–6.

60. Chatrath V, Banerjee PS, Goodman GJ, Rahman E. Soft-tissue filler-associated blindness: a systematic review of case reports and case series. Plast Reconstr Surg Glob Open. 2019; 7:e2173.

61. Liu C, Cai Z, Zhang L, Zhou M, He L. Case report and literature review: catastrophic embolism following cosmetic injection of autologous fat in the face. Front Med (Lausanne). 2021; 8:646657.
62. Tansatit T, Apinuntrum P, Phetudom T. Periorbital and intraorbital studies of the terminal branches of the ophthalmic artery for periorbital and glabellar filler placements. Aesthetic Plast Surg. 2017; 41:678–88.

63. Allali J, Bernard A, Assaraf E, Bourges JL, Renard G. Multiple embolizations of the branches of the ophthalmic artery: an unknown serious complication of facial surgeries. J Fr Ophtalmol. 2006; 29:51–7.
64. Dettmer MS, Willi N, Thiesler T, Ochsner P, Cathomas G. The impact of pulmonary bone component embolism: an autopsy study. J Clin Pathol. 2014; 67:370–4.

65. Park SM, Kim KH, Yoon NB, Jeong IH, Lee HW, Lee SK, et al. Clinical manifestations of 6 cases of septic pulmonary embolism at increased risk recently. Kosin Med J. 2012; 27:99–103.

66. Kim HS. A case of acute pulmonary embolism with a large right ventricular thrombus. Kosin Med J. 2009; 24:207–10.
Fig. 1.
The lung of a rabbit with experimentally induced fulminant-acute fat embolism syndrome, showing variable-sized small and large fat globules in the vessels of inter-alveolar septa with no remarkable change of the alveolar septa (Sudan Black B stain, ×100).
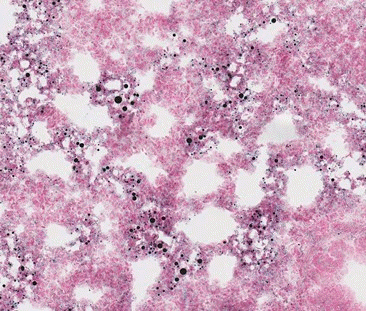
Fig. 2.
The lung of a rabbit with experimentally induced subacute fat embolism syndrome, showing many variable-sized black fat globules in the lung and some vascular spaces with diffuse hemorrhage in intra-alveolar spaces (Sudan Black B stain, ×100).
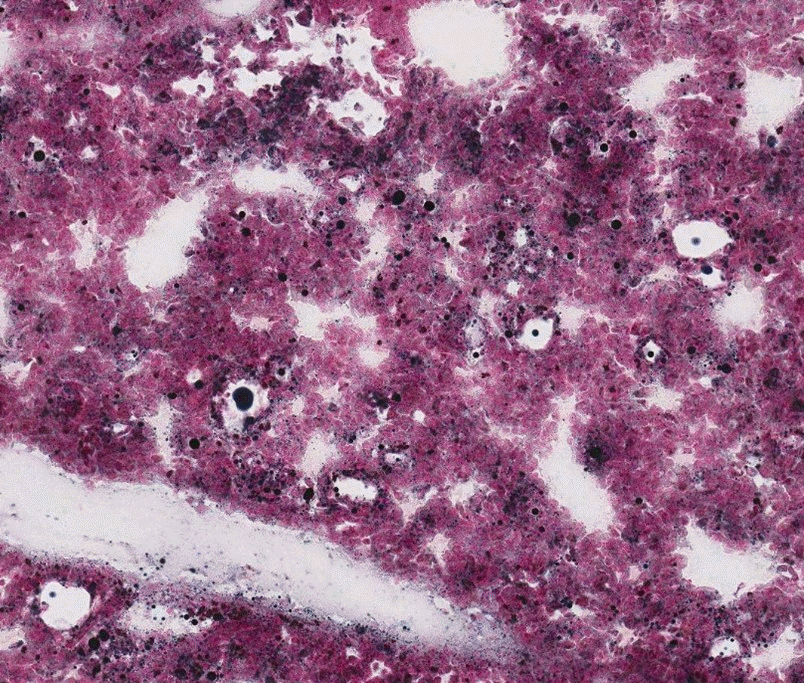
Table 1.
Various diagnostic criteria of fat embolism syndrome
Table 2.
Takahashi grading of cerebral fat embolism




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download