This article has been
cited by other articles in ScienceCentral.
Abstract
Background
This study aimed to elucidate the potential usefulness of the medial rectus muscle of cadavers for research on satellite cells.
Methods
Twenty-four medial rectus muscles were obtained from 12 cadavers. The control group included six medial rectus muscles from three live adults without brain activity. The muscle fiber diameter and distribution of satellite cells were measured and compared. Immunohistochemistry for myosin heavy chain and the transcription factor PAX7 was performed, and the distributions of myocytes and satellite cells were evaluated.
Results
The average muscle fiber diameter was 142.18±36.49 μm in the cadaver group and 149.34±15.26 μm in the control group, and there was no significant difference between the two groups (p=0.38). The ratio of PAX7(+) cells to the number of muscle fibers was 0.056±0.015 in the control group and 0.006±0.006 in the cadaver group, reflecting a significant difference (p<0.05).
Conclusions
The medial rectus muscles of cadavers can be helpful in studying anatomical morphology; however, their usefulness in muscle satellite cell research appears to be limited.
Go to :

Keywords: Cadaver, Myogenic satellite cell, Oculomotor muscles, PAX7 transcription factor
Introduction
In studies with human tissues, obtaining specimens from a living control group is difficult. Especially the needed tissues are from children, they are hardly able to obtain. One of the alternatives to such research is the cadaver study. Research on anatomical structure, muscles, nerves, and satellite cells using cadavers has been conducted [
1-
6]. In medical education, the reliability of cadavers as a suitable gross anatomy educational source is widely accepted; but the use of cadavers for histopathological studies is generally limited [
7,
8]. For extraocular muscles, low prevalence and difficulty in gathering specimens have resulted in the publication of only a few studies. Some reports using extraocular muscles from cadaver tissues have shown muscular and neural structures and are suggest promising methods for studying normal structures [
4-
6]. We focused on the extraocular muscle that might cause the strabismus. Satellite cell (PAX7) distribution could be a scale of myogenic ability.
It is reported that satellite cells in skeletal muscles maintain their myogenic capacity even when muscles are grafted 11 days postmortem [
9]. In the present study following the structure of medical science research [
10], we investigated satellite cell distribution using the medial rectus muscle of cadavers. The purpose of this study was to determine the potential usefulness of cadaver tissues in research by comparing cadaveric samples with those from living adults.
Go to :

Methods
Ethical statements: This study was approved by the Kosin University Gospel Hospital Institutional Review Board (IRB No. 2020-03-033), and the requirement for patient consent was waived.
1. IRB/IACUC approval
Prior to initiation of the study, the protocol was submitted to a qualified research ethics committee for review and approval [
11]. After approval from the Kosin University Gospel Hospital Institutional Review Board, we resected cadaveric medial rectus muscles and sent them to the Kosin BioBank. The control group specimens were collected from three brain-dead men who consented to organ donation. This study adhered to the principles of the Declaration of Helsinki and the Health Insurance Portability and Accountability Act. Appropriate informed consent and approvals were obtained from the family members before using the specimens.
2. Description of participants
Cadavers for study were donated for medical education of students in the first semester of 2020. From these 12 cadavers, we harvested 24 medial rectus muscles as a study group. The cadaveric subjects had no history of extraocular muscle or orbital disorders or eyelid or eye muscle surgery or trauma. As a control group, six medial rectus muscles were collected from three living men without brain function or history of eye disease.
3. Preparation, staining, and analysis
We used medial rectus muscles not including tendons at least 3 mm from the attachment site. After tissue harvesting, fixation, dehydration, and infiltration, paraffin blocks were made and cut into 4 µm sections. These sections were stained with hematoxylin (Poly Scientific) and eosin (Poly Scientific) and with immunochemical stains for myosin heavy chain (MyoHC) and PAX7. The average muscle bundle diameter of the extraocular muscle per unit area (320×240 μm) was obtained under an optical microscope. The ratio of the number of PAX7(+) cells to the total number of muscle bundles per unit area (320×240 μm) was calculated under a fluorescence microscope.
4. Statistics
The results of the two groups were compared using GraphPad Prism version 7.1 (GraphPad Software), and p<0.05 was considered statistically significant.
Go to :

Results
1. General characteristics
The mean age of the control group was 22.00±5.65 years, that of the cadaver group was 63.45±6.16 years, and the male: female ratios were 3:0 and 7:5, respectively.
2. Myofiber diameter
Optical microscopic examination with hematoxylin and eosin staining indicated that the mean myofiber diameter was 149.34±15.26 μm in the control group and 142.18±36.49 μm in the cadaver group. The difference was not significant (
p=0.38) (
Fig. 1).
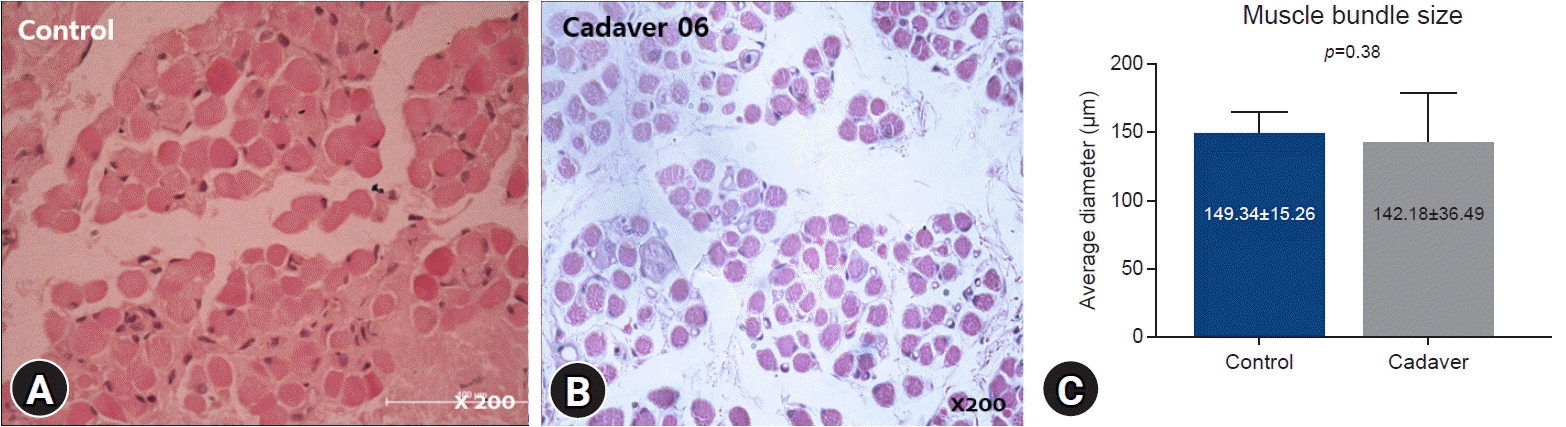 | Fig. 1.(A, B) Light microscope photographs. The medial rectus muscles of the control and cadaver groups showed similar average muscle bundle diameters (hematoxylin and eosin stain, ×200). (C) Muscle bundle diameter distributions are not significantly different between the two groups. 
|
3. PAX7 distribution
PAX7(+) satellite cells were identified by fluorescence microscopy, and the ratio of the number of positively stained nuclei to the number of observed muscle fibers was determined (
Fig. 2). The proportion of PAX7(+)/number of muscle fibers (
Figs. 3,
4) of the control group and the cadaver group was 0.056±0.015 and 0.006±0.006, respectively (
p<0.001) and was significantly greater in the control group.
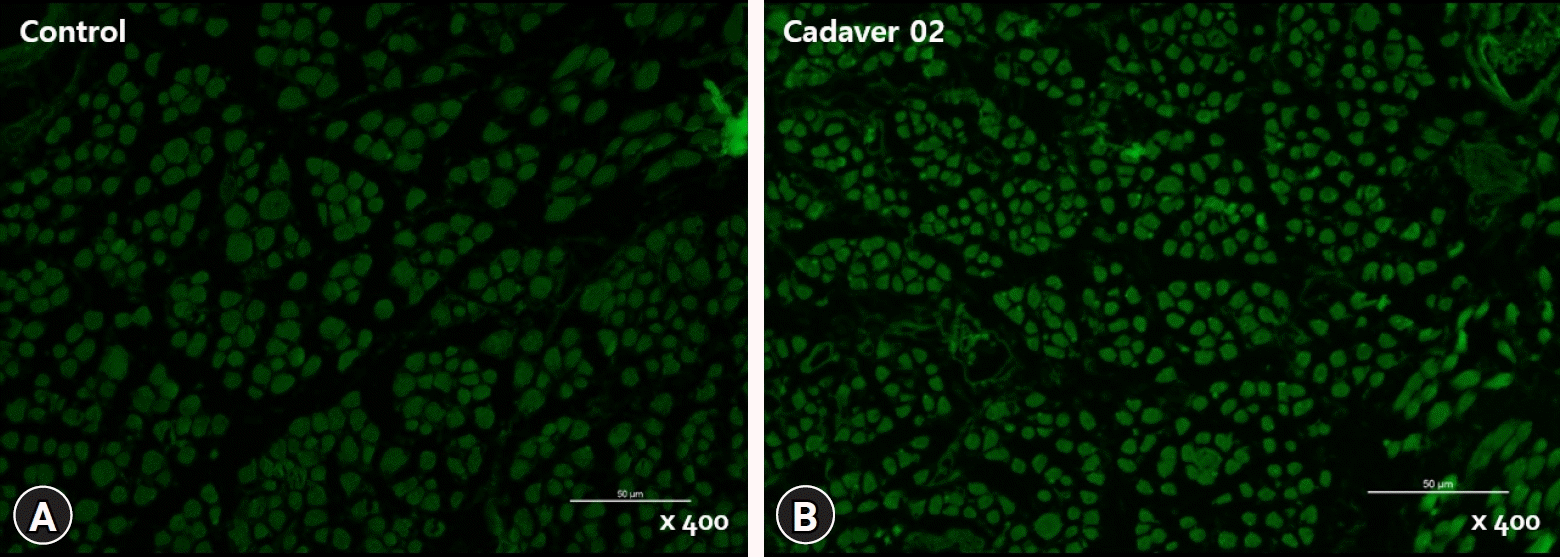 | Fig. 2.Fluorescence microscopic photographs of the medial rectus muscles of the control (A) and cadaver (B) groups; immunohistochemistry was performed (myosin heavy chain, ×400). 
|
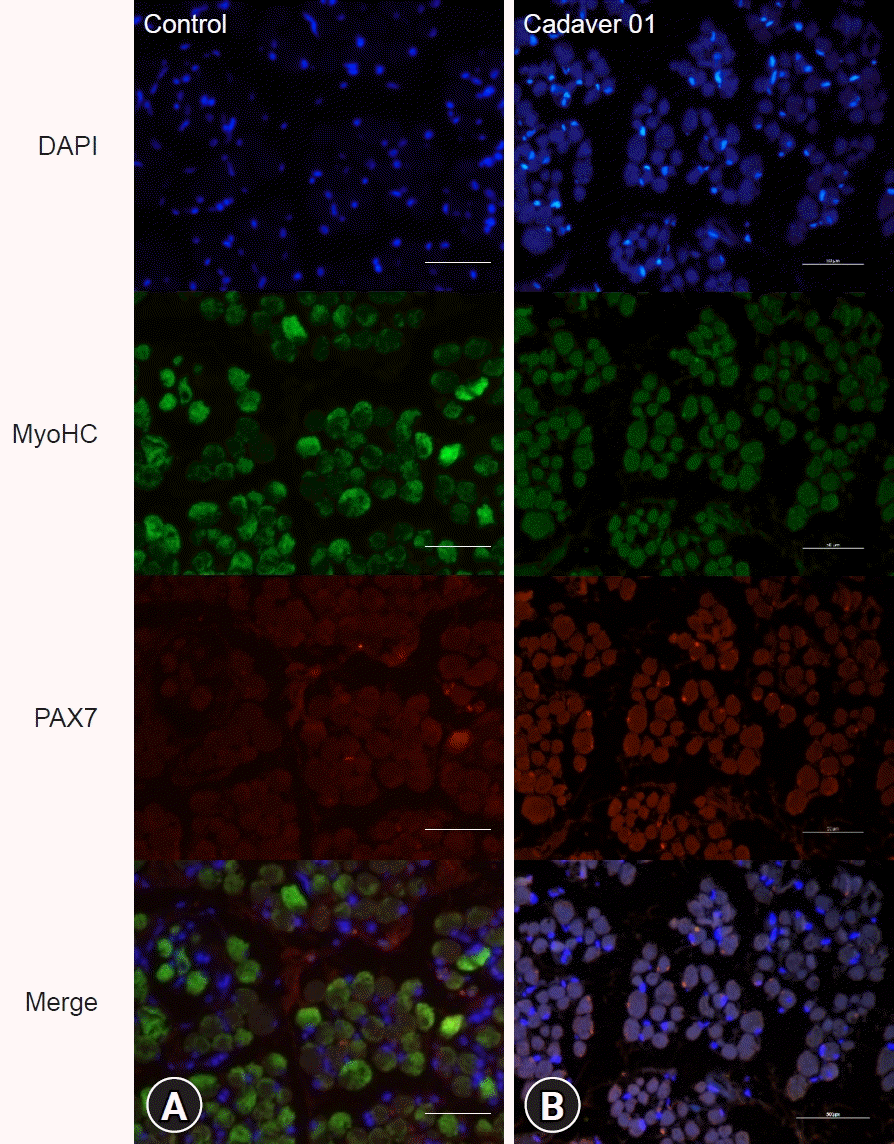 | Fig. 3.Fluorescence microscopy images of medial rectus muscles with immunohistochemistry of the control (A) and cadaver (B) groups. DAPI, 4′,6-diamidino-2-phenylindole; MyoHC, myosin heavy chain. 
|
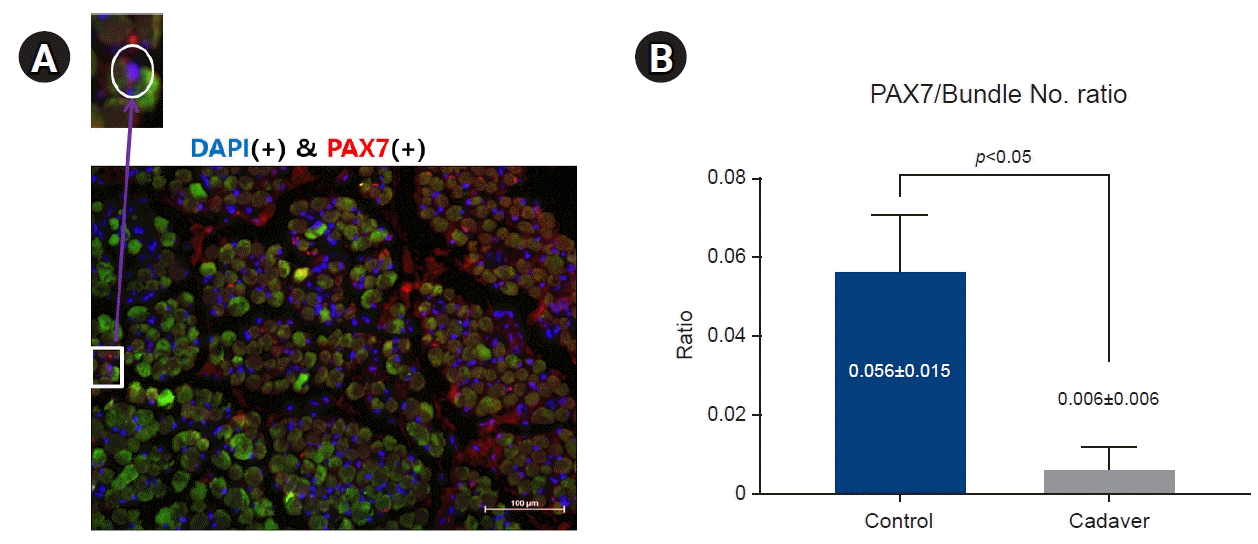 | Fig. 4.DAPI (+) and PAX7 (+) cells were found at the margin of the muscle fiber (A), and the ratio of the PAX7 (+) cells to the number of muscle fibers was higher in the control group than in the cadaver group (p<0.05) (B). DAPI, 4′,6-diamidino-2-phenylindole; MyoHC, myosin heavy chain. 
|
Go to :

Discussion
In our study, the mean myofiber diameters of the medial rectus muscles were not significantly different between the cadaver and control groups. However, the proportion of PAX7(+)/number of muscle fibers was significantly lower in the cadaver group than in the control group.
Our results regarding myofiber structure are different from those of a previous study. Latil et al. [
12] reported that skeletal muscle biopsies from human cadavers at 6 to 17 days postmortem showed severe autolytic alterations with edema compared with normal muscle. However, in our study, the structures of muscle bundles in the medial rectus were preserved. One possible reason for the inconsistency is the embalming process. Paik and Shin [
4] compared fresh and embalmed cadavers and reported that the embalming process could influence the diversity of investigations regarding inferior oblique muscle anatomy. The postmortem preservation of two types of muscles might not be the same because of the embalming process.
The number of satellite cells per fiber was relatively lower (0.056±0.015) than that in previous studies. Lindstrom et al. [
13] reported that the mean number of PAX7(+)/muscle fibers was 0.076±0.022 in the anterior portion of the extraocular muscles. In the present study, we also used the anterior portion of the medial rectus muscle as a control. The cadaver showed lower PAX7(+)/number of muscle fibers (0.0056±0.102). Other research studies reported that the myofibers with PAX7(+) were between 7% and 8% [
14]. Our study used only the medial rectus and used a different portion of PAX7(+) cells; while the previous study used superior, medial, and lateral rectus muscles. This may have accounted for the difference. Another possible explanation for the difference is alignment of the eyes. In a previous study with exotropia patients, the PAX7(+)/number of muscle fibers was lower than normal [
15]. In addition, postmortem periods were not the same, and postmortem stretching of the medial rectus muscles in some cadavers may have occurred due to embalming processes. Finally, since the number of studies and sample numbers in those studies are low, each data entry may alter the ratio significantly. These findings elucidate the need for a standard cadaver model for study.
Novak et al. [
9] developed a model to evaluate the maintenance of satellite cell function and myogenic capacity using human skeletal muscle and demonstrated preservation up to 11 days postmortem. In this medial rectus muscle study of cadavers, the myogenic capacity, especially in satellite cells (PAX7), was decreased. It was difficult to compare extropian differences because extraocular muscles at least 6 months postmortem and in an embalmed state. Also, the other reason is skeletal muscles not having undergone an embalming process [
9]. The factors of time and embalming process may combine to generate observational differences, but we can conclude that embalmed cadavers are not suitable sources for satellite cell distribution studies.
The present study has several limitations. First, it was conducted on only the medial rectus muscle. Because the aim of this experiment is to elucidate the potential usefulness of cadaver tissue as a research material, we did small, rapid project with medial rectus muscle. Second, because all cadavers are donated for medical education, the age and postmortem intervals could not be standardized. We could not compare the two groups by age or gender. Despite these limitations, this study has some strengths, including prospective tissue collection, significantly different results between the two groups, and definite conclusions.
In conclusion, there was no difference in the size of the medial rectus muscle fibers between cadavers and normal adults, but the proportion of stem cells was significantly reduced in the cadaver group. We suggest that the medial rectus muscle of cadavers has limited usefulness in anatomic research.
Go to :






 PDF
PDF Citation
Citation Print
Print






 XML Download
XML Download