Abstract
Remimazolam is a promising drug for general anesthesia due to its rapid onset, short duration, and short context-sensitive half-life. However, its use in pediatric patients remains off-label, and limited prospective data have been published. Herein, we report successful anesthesia using remimazolam in pediatric patients who had a history of epilepsy or required shared airway surgery. In all cases, remimazolam provided general anesthesia, and flumazenil was used for reversal with rapid recovery. Remimazolam offers advantages for pediatric anesthesia in scenarios with a risk of seizure or shared airway surgery. However, the potential for higher bispectral index values and the risk of anaphylaxis in dextran-allergic patients necessitate caution and further research.
Remimazolam (Byfavo; Hana Pharm Co., Ltd.) is a drug belonging to the benzodiazepine class, and rapidly metabolized via non-specific tissue esterases [1]. Remimazolam exhibits high clearance, ensuring a rapid onset and short duration of sedative action. This property provides predictable sedation across a broad dose range without causing cardiovascular or respiratory depression. Remimazolam does not cause pain upon injection, produces predictable levels of sedation, and has a reversal agent, making it a suitable option for sedation or general anesthesia in pediatric patients [2]. These advantages have led Horikoshi et al. [3] to the utilization of remimazolam for surgical anesthesia in pediatric patients with Duchenne muscular dystrophy who has the risk of malignant hyperthermia, and hemodynamic instability.
However, it is important to note that remimazolam is currently approved exclusively for use in adults, and there is a lack of prospective studies involving pediatric patients. In this report, the authors aim to share the experience with four patients for whom remimazolam was successfully employed for total intravenous anesthesia (TIVA), each for various reasons.
Ethical statements: This report was approved by the Institutional Review Board of Pusan National University Yangsan Hospital (IRB No. 05-2023-125), and the need for informed consent was waived due to the retrospective nature of the study.
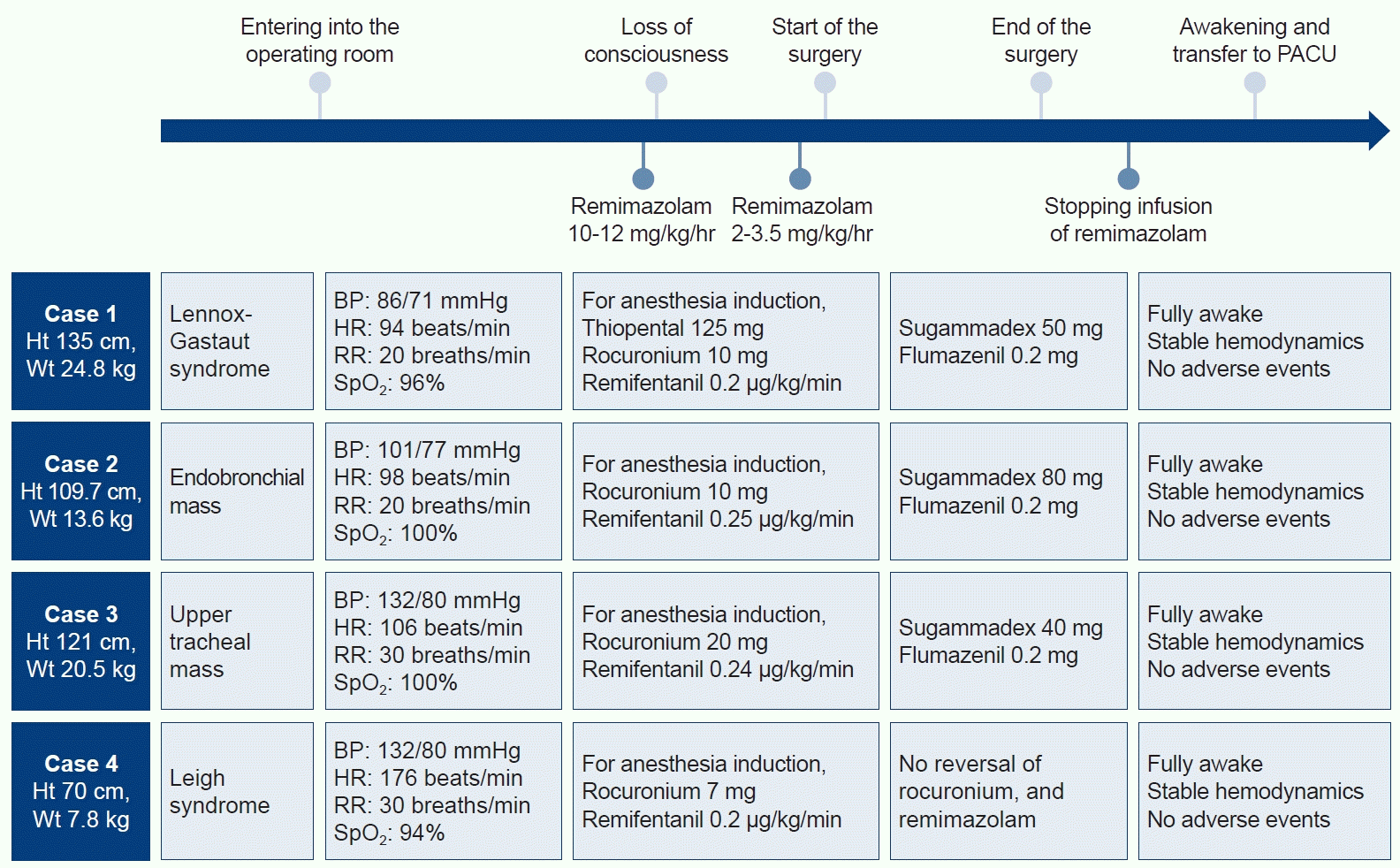
A 10-year-old boy (height: 135 cm; weight: 24.8 kg) with Lennox-Gastaut syndrome was admitted to our emergency department with increased respiratory secretions and percutaneous oxygen saturation (SpO2) below 80%. Aspiration pneumonia was diagnosed, and antibiotics were administered. One month later, the patient was scheduled for a tracheostomy. Due to the high risk of perioperative seizures in the patient and the availability of reversal agents after general anesthesia, it was planned to maintain anesthesia with remimazolam. Initial vital signs were as follows: blood pressure (BP) 86/71 mmHg, heart rate (HR) 94 beats per minute (bpm), respiratory rate (RR) 20 breaths/min, and SpO2 96%. A single bolus of thiopental 125 mg and rocuronium 10 mg were injected intravenously to induce anesthesia, and remimazolam was immediately administered only as a maintenance dose at a rate of 1.2 mg/kg/hr since thiopental was used for anesthesia induction, and remifentanil at a rate of 0.2 μg/kg/min was started. An endotracheal tube (ETT) was inserted, and surgery proceeded with the infusion of remimazolam at a rate of 2.0 mg/kg/hr and remifentanil at a rate of 0.07 μg/kg/min to maintain anesthesia. Bispectral index (BIS), neuromuscular transmission monitoring, and vital signs were continuously measured, with noninvasive BP checked every 5 minutes. Overall, the infusion rate remained consistent. The surgery took approximately 40 minutes. After the surgery was completed, remimazolam was halted immediately, the ETT was extubated, and a tracheostomy tube was placed. Flumazenil (Flunil; Bukwang Pharm Co., Ltd.) 0.2 mg and sugammadex (Bridion; Merck & Co., Inc.) 50 mg were administered intravenously. Five minutes later, the patient was quietly recovering from anesthesia without agitation and was transferred to the pediatric intensive care unit (Fig. 1).
A 5-year-old girl (height: 109.7 cm; weight: 13.6 kg) had a 0.6 cm-sized endobronchial mass in the left upper lobe, and rigid bronchoscopy was scheduled for the removal of the mass and histologic examination. Since the insertion of a rigid bronchoscope was necessary for the removal of the endobronchial mass, and there was a need to maintain spontaneous breathing, TIVA with remimazolam was chosen to avoid the leakage of inhalational anesthetic from a breathing circuit. She was monitored using standard monitors, and initial vital signs were as follows: BP 101/77 mmHg, HR 98 bpm, RR 20 breaths/min, and SpO2 100%. High-flow oxygen was applied, followed by the infusion of remimazolam at a rate of 11 mg/kg/hr and remifentanil at a rate of 0.25 μg/kg/min for a few minutes to induce anesthesia. After 30 minutes of anesthesia, 10 mg of rocuronium was administered intravenously as a moderate neuromuscular block was required for the surgical procedure. During the surgery, the oxygen reserve index (Masimo Corp.) measured using a sensor (rainbow sensor, R2–25, Revision L, Masimo Corp.) was monitored for the early detection of desaturation under tubeless anesthesia. When desaturation seemed to occur, the surgical instruments were removed from the trachea, and rescue mask ventilation was performed until the oxygen reserve index increased to around 1.0. For the maintenance of anesthesia, the rate of remimazolam infusion was maintained between 2.5 mg/kg/hr and 3.5 mg/kg/hr while monitoring the BIS, and remifentanil was administered at a rate of 0.37 μg/kg/min. Considering that the BIS may be slightly high during general anesthesia using remimazolam, the remimazolam administration dose was adjusted to maintain the BIS around 60–70. The surgery took approximately 75 minutes. Without hemodynamic instability, 0.2 mg of flumazenil and 80 mg of sugammadex were administered intravenously after the end of surgery. Spontaneous breathing was quickly restored within 5 minutes after discontinuing remimazolam, and the patient was then transferred to the post-anesthesia care unit (Fig. 1).
A 6-year-old girl (height: 121 cm; weight: 20.5 kg) was scheduled for ventilating bronchoscopy with a rigid bronchoscope because a polyp in the upper trachea was observed on a computed tomography scan due to increased wheezing. It was decided to proceed with the operation without inserting the ETT because the insertion of a rigid bronchoscope was necessary for the removal of the upper tracheal mass. TIVA with remimazolam was chosen in anticipation of a lower risk of leakage compared to volatile anesthetics. Initial vital signs were as follows: BP 132/80 mmHg, HR 106 bpm, RR 30 breaths/min, and SpO2 100%. Vital signs were continuously monitored, and noninvasive BP was checked every 5 minutes. To induce anesthesia, the attending anesthesiologist started with an infusion of remimazolam at a rate of 10 mg/kg/hr and remifentanil at a rate of 0.24 μg/kg/min with high-flow oxygen application. After she was sedated, 20 mg of rocuronium was administered. Anesthesia was maintained with an infusion of remimazolam at a rate of 2.4 mg/kg/hr and remifentanil at a rate of 0.33 μg/kg/min. When oxygen reserve index reached 0.2, an ETT was inserted into the trachea through the rigid bronchoscope, and the ETT was inserted through a rigid bronchoscope 5 to 6 times for ventilation. When oxygen reserve index increased to near 1.0, the operation was resumed. The surgery took approximately 120 minutes. Since ventilation was not performed during surgery, transcutaneous carbon dioxide (TcCO2; Sentec AG) was monitored instead of end-tidal carbon dioxide. After the surgery, an i-gel (Intersurgical Ltd) was placed to remove carbon dioxide before waking the patient up. TcCO2 rose to a maximum of 46.7 mmHg, and was 43.6 mmHg after ventilation for about 15 minutes. Intravenous flumazenil 0.2 mg and sugammadex 40 mg were administered, and after 5 minutes, the i-gel was removed. The patient was then taken to the post-anesthesia care unit (Fig. 1).
A 16-month-old girl (height: 70 cm; weight: 7.8 kg) with Leigh’s syndrome received cardiopulmonary resuscitation for cyanosis 1 month before the surgery. After the recovery of spontaneous circulation, she was mechanically ventilated. Due to repeated ventilator weaning failures, she was scheduled for a tracheostomy. Propofol and thiopental can cause mitochondrial dysfunction in patients with Leigh’s syndrome [4], and there is a rare risk of malignant hyperthermia. Therefore, the attending anesthesiologist decided to use remimazolam. The patient was intubated with an ETT (internal diameter 3.5 mm) and entered the operating room. She was monitored using standard monitors, and initial vital signs were as follows: BP 132/80 mmHg, HR 176 bpm, RR 30 breaths/min, and SpO2 94%. For the induction of anesthesia, an infusion of remimazolam at a rate of 10 mg/kg/hr was started. After she became sedated, 7 mg of rocuronium was administered, and the infusion rate of remimazolam was maintained at 2.5 mg/kg/hr. The surgery took approximately 30 minutes. After the surgery, the ETT was extubated, and a tracheostomy tube was placed. She recovered from anesthesia without agitation and was transferred to the pediatric intensive care unit (Fig. 1).
The authors successfully performed TIVA with remimazolam in pediatric patients who were expected to experience a perioperative seizure and in other pediatric patients who required TIVA due to airway surgery, where maintaining an adequate depth of anesthesia with inhalational anesthetics was challenging. The characteristics of remimazolam, which prevent seizures and have less impact on the patient’s hemodynamic stability and spontaneous respiration made it suitable for general anesthesia in these clinical situations [5]. There have been studies in which remimazolam was used for the general anesthesia, or reducing postoperative excitement in children, and it was conducted without critical complications [6,7].
Remimazolam, being a benzodiazepine, possesses anticonvulsant properties [8,9]. Using a benzodiazepine in conditions where seizures are common, especially one with a short context-sensitive half-life like remimazolam, offers clear advantages in anesthesia [5]. According to a report by Yamadori et al. [9], general anesthesia using remimazolam was performed safely without seizures in pediatric patient with mitochondrial disease. Thiopental, which can also be used as an anticonvulsant, is unsuitable for prolonged sedation due to its long context-sensitive half-life. In the study conducted on 20 healthy volunteers by Eisenried et al. [10], remimazolam showed the characteristics of high clearance, small steady-state volume of distribution, and short terminal phase half-life, with a short context-sensitive half time of approximately 6.8±2.4 minutes. Predicting the patient's awakening is easier with remimazolam compared to inhalational anesthetics, and the presence of a reversal agent such as flumazenil further enhances this advantage.
Although remimazolam is a drug known for its ultrashort-acting properties, the recovery of consciousness after general anesthesia has been reported to be slower with remimazolam than with propofol [11]. Flumazenil is a reversal agent of benzodiazepine that can be used to reverse remimazolam or to control the level of sedation caused by remimazolam. While concerns regarding re-sedation after flumazenil reversal exist, Lee et al. [12] reported that the planned administration of flumazenil following remimazolam-based TIVA resulted in rapid recovery of consciousness, with a time to eye opening of 2.3 minutes and time to extubation of 3.2 minutes. When reversing benzodiazepine-induced sedation in children, the recommended starting dose of flumazenil is 0.01 mg/kg (maximum 0.2 mg), and it should be administered intravenously over 15 seconds [13]. The risk of seizures caused by flumazenil is known to be very low, but caution is required to prevent convulsions during administration [14].
In shared airway surgeries requiring the placement of a rigid bronchoscope, ventilation becomes challenging when connecting a breathing circuit through the side port of the bronchoscope. Additionally, using inhalation anesthetics leads to leakage, making it difficult to maintain the appropriate depth of anesthesia. In these cases, mechanical ventilation was initiated by connecting a breathing circuit to the rigid bronchoscope. However, virtually all of it leaked through the cracks, making it difficult to maintain anesthesia with inhalation anesthetics. TIVA allowed for repeated rescue ventilation while maintaining a stable depth of anesthesia. Nevertheless, TIVA is typically performed using propofol, which has several limitations in children. For instance, it can be painful when injected intravenously and poses a risk of propofol-related infusion syndrome in pediatric patients. Thus, using propofol for anesthesia in children under 3 years of age remains off-label. Despite its widespread use in pediatric anesthesia, there is conflicting literature regarding its safety [15]. On the other hand, remimazolam can be a useful option for TIVA in children because remimazolam is painless to inject and is associated with less hypotension, bradycardia, and respiratory depression compared to propofol [3].
However, it is important to note several limitations and cautions of using remimazolam in pediatric patient. The use of remimazolam for anesthesia in children is still considered off-label. There are only a few case reports of remimazolam use in children, and no prospective studies, pediatric pharmacokinetic/pharmacodynamics studies, or criteria for effect-site concentration in children, indicating the need for further research. The data on the pharmacokinetics of remimazolam has not yet obtained, and the recommended maintenance dose for remimazolam is 1–2 mg/kg/hr typically [16]. However, research conducted by Doi et al. [11] showed that BIS values during general anesthesia with remimazolam were higher compared to propofol, and Fang et al. [2] conducted a preliminary study on pediatric patients receiving general anesthesia with remimazolam, where they observed that the average BIS value consistently exceeded 60 throughout the anesthesia induction and maintenance period. In these cases, it was necessary to administer remimazolam at a rate of more than 2 mg/kg/hr to maintain the depth of anesthesia properly. While the exact prevalence of anaphylaxis caused by remimazolam remains unknown, there have been reported cases of anaphylaxis and circulatory collapse associated with its use [17,18]. Remimazolam should be avoided in patients allergic to dextran or dextran-containing products because dextran 40, an additive in remimazolam, may be a potential trigger for anaphylactic reactions [18].
In conclusion, the characteristics of benzodiazepine drugs, along with this particular case, suggest that remimazolam could be alternative for patients with a history of seizures. In addition, TIVA using remimazolam is a method that can achieve an appropriate depth of anesthesia during shared airway surgery even if there is air leakage. For pediatric cases, when there's hesitance towards using propofol due to concerns such as pain, propofol infusion syndrome, or mitochondrial dysfunction, remimazolam emerges as a viable alternative. However, there are several limitations and precautions associated with its use in pediatric patients, including the need for further research, the potential for higher BIS values.
Notes
References
1. Kim KM. Remimazolam: pharmacological characteristics and clinical applications in anesthesiology. Anesth Pain Med (Seoul). 2022; 17:1–11.
2. Fang YB, Wang CY, Gao YQ, Cai YH, Chen J, Zhang XL, et al. The safety and efficacy of remimazolam tosylate for induction and maintenance of general anesthesia in pediatric patients undergoing elective surgery: study protocol for a multicenter, randomized, single-blind, positive-controlled clinical trial. Front Pharmacol. 2023; 14:1090608.

3. Horikoshi Y, Kuratani N, Tateno K, Hoshijima H, Nakamura T, Mieda T, et al. Anesthetic management with remimazolam for a pediatric patient with Duchenne muscular dystrophy. Medicine (Baltimore). 2021; 100:e28209.

4. Niezgoda J, Morgan PG. Anesthetic considerations in patients with mitochondrial defects. Paediatr Anaesth. 2013; 23:785–93.

5. Kim SH, Fechner J. Remimazolam: current knowledge on a new intravenous benzodiazepine anesthetic agent. Korean J Anesthesiol. 2022; 75:307–15.

6. Petkus H, Willer BL, Tobias JD. Remimazolam in a pediatric patient with a suspected family history of malignant hyperthermia. J Med Cases. 2022; 13:386–90.
7. Kimoto Y, Hirano T, Kuratani N, Cavanaugh D, Mason KP. Remimazolam as an adjunct to general anesthesia in children: adverse events and outcomes in a large cohort of 418 cases. J Clin Med. 2023; 12:3930.

8. Kilpatrick GJ. Remimazolam: non-clinical and clinical profile of a new sedative/anesthetic agent. Front Pharmacol. 2021; 12:690875.

9. Yamadori Y, Yamagami Y, Matsumoto Y, Koizumi M, Nakamura A, Mizuta D, et al. General anesthesia with remimazolam for a pediatric patient with MELAS and recurrent epilepsy: a case report. JA Clin Rep. 2022; 8:75.

10. Eisenried A, Schuttler J, Lerch M, Ihmsen H, Jeleazcov C. Pharmacokinetics and pharmacodynamics of remimazolam (CNS 7056) after continuous infusion in healthy male volunteers: part II. pharmacodynamics of electroencephalogram effects. Anesthesiology. 2020; 132:652–66.
11. Doi M, Morita K, Takeda J, Sakamoto A, Yamakage M, Suzuki T. Efficacy and safety of remimazolam versus propofol for general anesthesia: a multicenter, single-blind, randomized, parallel-group, phase IIb/III trial. J Anesth. 2020; 34:543–53.
12. Lee HJ, Lee HB, Kim YJ, Cho HY, Kim WH, Seo JH. Comparison of the recovery profile of remimazolam with flumazenil and propofol anesthesia for open thyroidectomy. BMC Anesthesiol. 2023; 23:147.

13. Mancuso CE, Tanzi MG, Gabay M. Paradoxical reactions to benzodiazepines: literature review and treatment options. Pharmacotherapy. 2004; 24:1177–85.

14. Nguyen TT, Troendle M, Cumpston K, Rose SR, Wills BK. Lack of adverse effects from flumazenil administration: an ED observational study. Am J Emerg Med. 2015; 33:1677–9.

15. Chidambaran V, Costandi A, D’Mello A. Propofol: a review of its role in pediatric anesthesia and sedation. CNS Drugs. 2015; 29:543–63.

16. Hu Q, Liu X, Wen C, Li D, Lei X. Remimazolam: an updated review of a new sedative and anaesthetic. Drug Des Devel Ther. 2022; 16:3957–74.
Fig. 1.
Timeline with vital signs, drugs, and major events. Except for patient 1, all three patients underwent induction and maintenance of anesthesia using remimazolam. When the patients woke up from general anesthesia, flumazenil and sugammadex were administered intravenously. PACU, post-anesthesia care unit; Ht, height; Wt, weight; BP, blood pressure; HR, heart rate; RR, respiration rate; SpO2, saturation of percutaneous oxygen.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download