Abstract
Although most children with coronavirus disease 2019 (COVID-19) infection present with mild symptoms, a few pediatric patients develop severe neurological manifestations. Herein, we describe the case of a pediatric patient who presented with rapidly progressive diffuse and fatal cerebral edema associated with COVID-19. A previously healthy 6-year-old boy was diagnosed with acute fulminant cerebral edema (AFCE), which resulted in transtentorial downward herniation within 48 hours after the initial onset of fever. Detailed history-taking, close monitoring of the consciousness level with serial neurological examinations, and prompt diagnosis and treatment are required in patients suspected to have AFCE. Further research is needed to identify the pathogenesis of AFCE associated with COVID-19 and the related risk factors.
Throughout the coronavirus disease (COVID-19) pandemic, pediatric patients typically present with no symptoms or symptoms milder than those in adults [1,2]. However, a severely poor prognosis, including hospitalization and death due to COVID-19, has been reported [3,4]. The most common symptoms are limited to the upper respiratory tract; however, few case series of severe neurologic manifestations have been recently reported in children [5,6]. Herein, we reported the fatal case of a previously healthy Korean child with COVID-19 who developed rapidly progressive diffuse cerebral edema that resulted in brain death.
A previously healthy 6-year-old boy was admitted to the emergency room (ER) with altered mental status, new-onset seizures, and fever for 12 hours. At that time, an omicron variant (B.1.1.529) pandemic occurred in the community, and the incidence of COVID-19 in children increased rapidly in Korea. Prior to hospitalization, the patient abruptly showed altered mental changes 10 hours after fever onset, and emergency medical services were called. Upon the arrival of the emergency medical team, the patient was found to be stuporous. The following vital signs were obtained: blood pressure of 87/47 mmHg, heart rate of 170 beats/min, and saturation of peripheral oxygen (SpO2) level of 70%. After the administration of oxygen through a reservoir bag mask, the SpO2 level recovered to 100%. The patient arrived at the ER 1.5 hours later. During transport to the hospital, he experienced intermittent convulsions in the ambulance. Upon arrival at the hospital, he presented with generalized tonic-clonic (GTC) seizures with eyeball deviation. The convulsions ceased following the intravenous administration of lorazepam. The body weight was 28 kg, while the body mass index was 18.2 kg/m2. He was born at term with a normal weight for gestational age and was one of a set of twins. He had no history of chronic illness. His vital signs upon arrival at the ER were as follows: blood pressure, 76/30 mmHg; heart rate, 180 beats/min; respiratory rate, 30 breaths/min; body temperature, 40.8 °C; and SpO2 level, 96%, while receiving supplemental oxygen therapy through a facial mask at a flow rate of 15 L/min. He received initial rapid intravenous hydration. The rapid antigen test and real-time polymerase chain reaction (RT-PCR) for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) yielded positive results. Twenty minutes later, another GTC seizure occurred and stopped soon after administration of lorazepam. On examination, the patient was capable of breathing on his own, and his pupils remained equal, round, and reactive to light. However, his Glasgow Coma Scale (GCS) was 8 (eye opening 1, verbal response 3, and motor response 4). There were also no signs of upper motor neuron dysfunction, such as hyperactive deep tendon reflex, ankle clonus, or positive Babinski sign, but both limbs seemed to be rigidity. His skin was cool, with no erythema, rash, or post-traumatic bruising. Electrocardiogram showed a normal sinus rhythm, while chest radiography revealed diffuse mild infiltration in both lung fields but with no cardiomegaly. The initial blood test findings were shown in Table 1. Inotropics and vasopressors were administered to treat fluid-refractory shock and methylprednisolone (2 mg/kg/day) and intravenous immunoglobulin (2 g/kg/day) to treat suspected multiorgan inflammation arising from COVID-19. Although his SpO2 level was >95% while on supplemental oxygen therapy, he remained stuporous and in shock even after the administration of inotropics and vasopressors. His GCS was 4 at that time. Brain computed tomography (CT) and lumbar puncture were planned when his vital signs stabilized. However, the patient suddenly vomited and aspirated, reducing his SpO2 level to 75% and his self-respiration was diminished, which then required emergent intubation. His pupils were fixed and dilated (7/7 mm), and any response was not observed to painful stimuli (30 hours after fever onset). He was administered with dexamethasone 0.6 mg/kg per day divided by 4 doses and mannitol 2 g/kg per day divided by 6 doses for increased intracranial pressure (IICP) and empirical antibiotics (vancomycin and cefotaxime). A non-contrast brain CT revealed obliteration of the sulci and cisternal spaces of the brain (Fig. 1A). There were no specific relevant findings on abdominal CT, while multifocal atelectasis was found on chest CT. He underwent a point-of-care cardiac ultrasound performed by a pediatric cardiologist, which demonstrated no biventricular dysfunction or mild ventricular septal thickening. Specific organisms associated with the disease were not present in the blood, sputum, or urine. The RT-PCR results for respiratory viruses and bacteria obtained from tracheal aspiration cultures were negative. Serological evidence of cytomegalovirus, herpes virus, and Epstein–Barr virus was absent. Lymphocyte subsets were within normal limits. Treatment with pulsed methylprednisolone was initiated because of a sustained coma state. Central diabetes mellitus developed after 12 hours of acute deterioration. He was transferred to a negative-pressure isolation room in the intensive care unit (ICU) 20 hours after ER admission. The Pediatric Index of Mortality 3 score was 2.31, and the probability was 10.08. Brain magnetic resonance imaging showed hyperintensities in the thalami, pons, and midbrain and severe cerebral edema with transtentorial downward herniation (Fig. 1B). Given his overall clinical features and laboratory findings, we diagnosed acute fulminant cerebral edema (AFCE) due to COVID-19. The pupillary, corneal, oculocephalic, gag, and cough reflexes were not present. Electroencephalogram showed no activity exceeding 2 μV at a sensitivity of 2 μV/mm, consistent with electrocerebral inactivity. These findings suggest brain death. His family was willing to donate the patient’s organs to value his life. The Korea Organ Donation Agency replied that a brain death investigation for organ donation could only be performed 21 days after COVID-19 diagnosis. Hemodynamic, respiratory, endocrine, and optimal fluid managements were performed to protect and optimize organ function as well as to avoid therapy-induced complications, such as ventilator-induced injury, ICU-associated infection, or excessive vasoconstriction. There were several episodes of hemodynamic instability due to vasoplegia or sympathetic storms. The GCS score was maintained at 3 points during ICU care. The positive RT-PCR results for SARS-CoV-2 were consistent, and the cycle threshold values are shown in Table 2. On hospital day 22, the patient underwent surgery for organ donation.
To our knowledge, this study is the first pediatric case report of AFCE associated with COVID-19 in Korea according to the case report guideline [8]. AFCE has been recently recognized as a rare but devastating phenotype of suspected encephalitis associated with other viral causes, which has a high mortality rate in children [9,10]. Clinicians in tertiary institutions likely encounter only one or two cases of AFCE every few years [11]. A previous study has reported that in 1,038 pediatric patients with encephalitis, the incidence of AFCE was 2.4%, with 64% of patients dying, 32% in a vegetative state, and 4% with severe neurologic deficits [12]. Our case satisfied the following diagnostic criteria for AFCE: fever, altered mental status, and/or new-onset seizures, followed by progression to diffuse cerebral edema as documented by neuroimaging and/or autopsy, without organic brain injury, metabolic disorder, or pre-existing neurological illness [10]. This case presentation is similar with few previous case reports of AFCE related to COVID-19 in pediatric patients. A retrospective study of neurologic involvement in 365 children and adolescents conducted in the United States reported that four previously healthy children had AFCE associated with COVID-19 or multisystem inflammatory syndrome [10]. Of these, three patients presented with status epilepticus, cerebral edema in imaging studies, and cardiac arrest within 24 hours of hospital admission and were diagnosed with brain death. Two additional fatal cases of COVID-19-associated AFCE also presented with seizure-related cerebral edema within 12 hours, unlike other etiologies observed in the United States [13,14].
The clinical features of AFCE generally include fever, altered sensorium, and seizures, similar to those of many pediatric encephalitis cases. One distinction that differentiates AFCE is the presence of IICP which present including headache, nausea, vomiting and altered mental status [11]. Early recognition of IICP and serial neurologic examination are important because its symptoms could be nonspecific. The most critical consequences of AFCE include intracranial hypertension, secondary brain injury due to hypoxia and/or ischemia, and herniation [11]. Few studies have investigated the pathogenesis and risk factors of AFCE. A retrospective study on AFCE reported that the mean duration from the onset of neurological symptoms to the presentation of the signs of brain herniation was less than 3 days [12]. In our case, the patient presented with signs of brain herniation more rapidly (within 12 hours). No specific treatment regimen was associated with improved AFCE outcomes. Lan et al. [12] recommended that if the medical management of IICP in patients with acute encephalitis fails, decompressive craniectomy may be considered. Herein, the patient did not undergo decompressive craniectomy, considering the risks and benefits of this procedure.
Among 46 pediatric patients with COVID-19 who had died by September 3, 2022 (fatal rate of 0.85/100,000), we first described the clinical course of fatal COVID-19 [15]. The risk factors among critically ill children with COVID-19 include heart disease, diabetes mellitus, obesity, and chronic lung diseases other than asthma and seizure disorders [16,17]. However, the present case findings showed that COVID-19 may cause AFCE in previously healthy children. Because the COVID-19 pandemic is unpredictable, and there may be an increase in the number of severely ill patients, this case report that presented a rapid and devastating clinical course would be helpful for medical staff in ERs and pediatricians. In our case, the decrease in consciousness progressed more abruptly, and the patient’s condition worsened rapidly compared with the typical course of encephalitis. Detailed history-taking, close monitoring of the consciousness level with serial neurologic examination using the GCS, and prompt diagnosis and treatment are required in patients with suspected AFCE. Further research is needed to identify the pathogenesis of AFCE associated with COVID-19 and the related risk factors.
Notes
References
1. Sisk B, Cull W, Harris JM, Rothenburger A, Olson L. National trends of cases of COVID-19 in children based on US State Health Department data. Pediatrics. 2020; 146:e2020027425.

2. Bhopal SS, Bagaria J, Olabi B, Bhopal R. Children and young people remain at low risk of COVID-19 mortality. Lancet Child Adolesc Health. 2021; 5:e12–3.
3. Liguoro I, Pilotto C, Bonanni M, Ferrari ME, Pusiol A, Nocerino A, et al. SARS-COV-2 infection in children and newborns: a systematic review. Eur J Pediatr. 2020; 179:1029–46.

4. Harwood R, Yan H, Talawila Da Camara N, Smith C, Ward J, Tudur-Smith C, et al. Which children and young people are at higher risk of severe disease and death after hospitalisation with SARS-CoV-2 infection in children and young people: a systematic review and individual patient meta-analysis. EClinicalMedicine. 2022; 44:101287.

5. O'Loughlin L, Alvarez Toledo N, Budrie L, Waechter R, Rayner J. A systematic review of severe neurological manifestations in pediatric patients with coexisting SARS-CoV-2 infection. Neurol Int. 2021; 13:410–27.
6. Schober ME, Pavia AT, Bohnsack JF. Neurologic manifestations of COVID-19 in children: emerging pathophysiologic insights. Pediatr Crit Care Med. 2021; 22:655–61.

7. Kliegman R, St. Geme JW III. Nelson textbook of pediatrics. Elsevier;2024.
8. Im SI. How to write case reports in medicine. Kosin Med J. 2022; 37:102–6.
9. Maeda K, Chong PF, Akamine S, Yamashita F, Morooka Y, Mori H, et al. Case report: acute fulminant cerebral edema with perivascular abnormalities related to Kawasaki disease. Front Pediatr. 2021; 9:732110.

10. LaRovere KL, Riggs BJ, Poussaint TY, Young CC, Newhams MM, Maamari M, et al. Neurologic involvement in children and adolescents hospitalized in the United States for COVID-19 or multisystem inflammatory syndrome. JAMA Neurol. 2021; 78:536–47.
11. Krishnan P, Glenn OA, Samuel MC, Sheriff H, Foster-Barber A, Sejvar JJ, et al. Acute fulminant cerebral edema: a newly recognized phenotype in children with suspected encephalitis. J Pediatric Infect Dis Soc. 2021; 10:289–94.

12. Lan SY, Lin JJ, Hsia SH, Wang HS, Chiu CH, Lin KL, et al. Analysis of fulminant cerebral edema in acute pediatric encephalitis. Pediatr Neonatol. 2016; 57:402–7.

13. Ninan S, Thompson P, Gershon T, Ford N, Mills W, Jewells V, et al. Fatal pediatric COVID-19 case with seizures and fulminant cerebral edema. Child Neurol Open. 2021; 8:2329048X211022532.
14. Kim MG, Stein AA, Overby P, Kleinman G, Nuoman R, Gulko E, et al. Fatal cerebral edema in a child with COVID-19. Pediatr Neurol. 2021; 114:77–8.

15. Shin E, Choe YJ, Ryu B, Kim NY, Lee HJ, Kim DH, et al. Pediatric deaths associated with coronavirus disease 2019 (COVID-19) in Korea. J Korean Med Sci. 2023; 38:e21.

Fig. 1.
Brain imaging findings. (A) Non-contrast brain computed tomography shows an obliteration of the sulci (red arrow) and cisternal spaces of the brain (yellow line). (B) Sagittal T1 brain magnetic resonance image shows hyperintensities in the thalami, pons, and midbrain and severe cerebral edema with transtentorial downward herniation (red arrow).
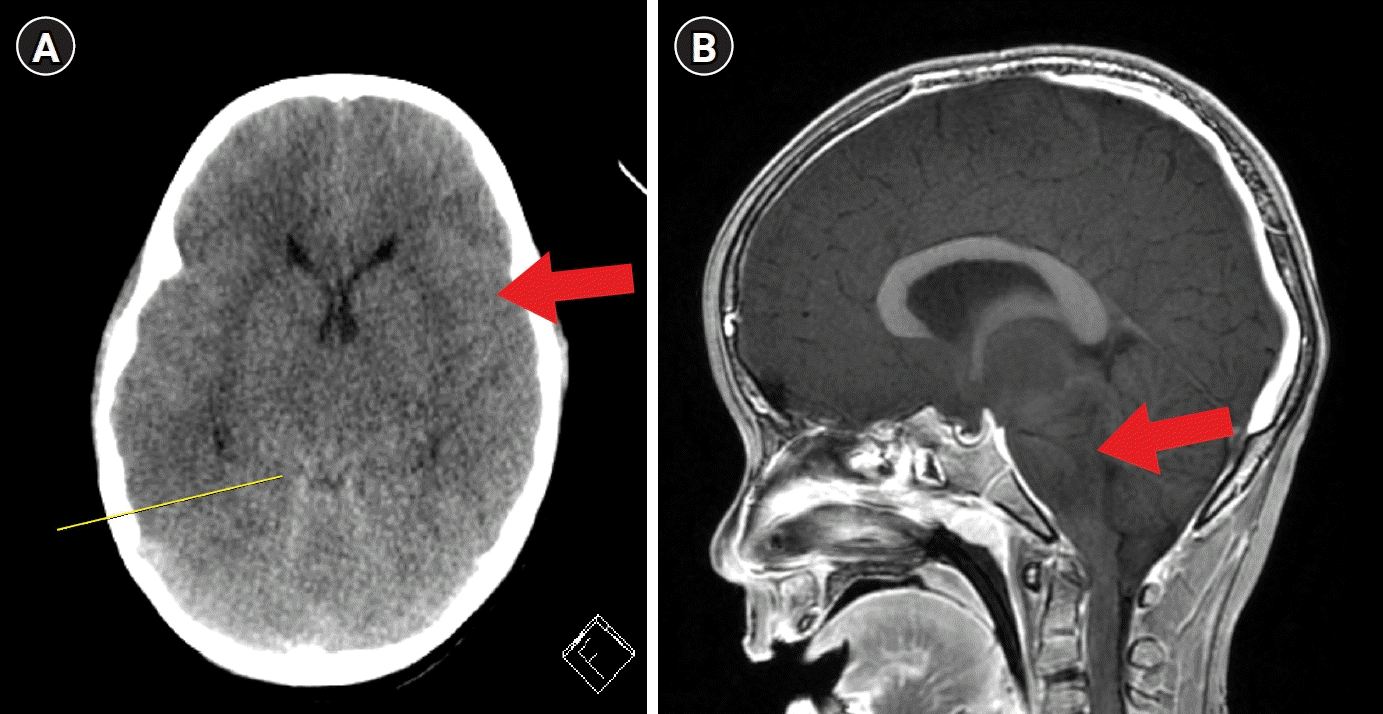
Table 1.
Initial laboratory data
PT/INR, prothrombin time/international normalized ratio; CRP, C-reactive protein; CPK, creatine phosphokinase.
a) Reference values are affected by many variables. The ranges for children aged 8 years were obtained from the second edition of the Nelson Textbook of Pediatrics [7].




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download