Abstract
Purpose
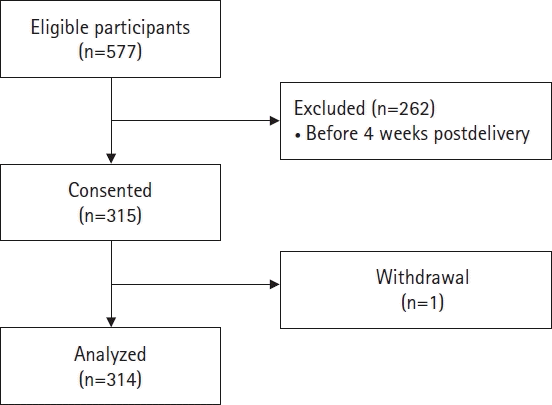
Methods
Results
Notes
Authors’ contributions
Conceptualization: Zangmo S, Boonchieng W, Suvanayos C, Siewchaisakul P; Data collection: Zangmo S; Formal analysis: Zangmo S, Siewchaisakul P; Writing–original draft: Zangmo S, Siewchaisakul P; Writing–review & editing: All authors.
Acknowledgments
Sherab Zangmo is a graduate student at Chiang Mai University (Faculty of Public Health) pursuing a master’s degree program under a scholarship from the Thailand International Cooperation Agency (TICA). The authors express profound gratitude. We would like to acknowledge Dr. Thinley Yangzom, Head of the Department and the staff of at the Gyaltsuen Jetsun Pema Wangchuck Mother and Child Hospital (GJPWMCH) with a special mention to the postnatal care unit for their invaluable support during the data collection phase. We would also like to express our sincere appreciation to all the participants.
Supplementary materials
Supplementary Table 1.
References
Table 1.
| Characteristics | Categories |
n (%) |
p-value | ||
|---|---|---|---|---|---|
| Total | Normal† | PPD‡ | |||
| Age (year)§ | <25 | 70 (22.29) | 55 (78.57) | 15 (21.43) | .126 |
| 25–34 | 195 (62.10) | 167 (85.64) | 28 (14.36) | ||
| >35 | 49 (15.61) | 45 (91.84) | 4 (8.16) | ||
| Education level | Primary school and below | 56 (17.83) | 53 (94.64) | 3 (5.36) | .064 |
| High school | 176 (56.10) | 144 (81.82) | 32 (18.18) | ||
| Others | 82 (26.11) | 70 (85.37) | 12 (14.63) | ||
| Religion | Buddhist | 269 (85.70) | 229 (85.13) | 40 (14.87) | .905 |
| Others | 45(14.33) | 38 (84.44) | 7 (15.56) | ||
| Ethnicity | Ngalong | 91(28.99) | 77 (84.62) | 14 (15.38) | .524 |
| Sharchop | 136 (43.31) | 112 (82.35) | 24 (17.65) | ||
| Lhotsam | 68 (21.66) | 61 (89.71) | 7 (10.29) | ||
| Others | 19 (6.05) | 17 (89.47) | 2 (10.53) | ||
| Currently living/address in Thimphu | No | 58 (18.48) | 47 (81.03) | 11 (18.97) | .345 |
| Yes | 256 (81.52) | 220 (85.94) | 36 (14.06) | ||
| Employment status of husband/partner (N=313) | No | 13 (4.14) | 11 (84.62) | 2 (15.38) | .966 |
| Yes | 300 (95.54) | 255 (85.00) | 45 (15.00) | ||
| Employment status of the participants | No | 174 (55.41) | 143 (82.18) | 31(17.82) | |
| Yes | 140 (44.59) | 124 (88.57) | 16 (11.43) | .115 | |
| Type of employment | Government job | 42 (13.38) | 36 (85.71) | 6 (14.29) | .387 |
| Private & co-operative | 50 (15.92) | 44 (88.00) | 6 (12.00) | ||
| Housewife & unemployed | 167 (53.18) | 137 (82.04) | 30 (17.96) | ||
| Others | 55 (17.52) | 50 (90.91) | 5 (9.09) | ||
| Monthly family income (Nu.) | <20,000 | 35 (11.15) | 24 (68.57) | 11 (31.43) | .015 |
| ≥20,000, <50,000 | 182 (57.96) | 159 (87.36) | 23 (12.64) | ||
| ≥50,000 | 97 (30.89) | 84 (86.60) | 13 (13.40) | ||
| Type of family | Nuclear | 150 (47.77) | 133 (88.67) | 17 (11.33) | .084 |
| Extended | 164 (52.23) | 134 (81.71) | 30 (18.29) | ||
Table 2.
Table 3.
| Characteristics | Categories |
n (%) |
p-value | ||
|---|---|---|---|---|---|
| Total | Normal | PPD | |||
| Number of living children | ≤1 | 154 (49.04) | 127 (82.47) | 27 (17.53) | .211 |
| ≥2 | 160 (50.96) | 140 (87.50) | 20 (12.50) | ||
| Nature of labor pain | Spontaneous | 149 (47.45) | 120 (80.54) | 29 (19.46) | .085 |
| Induced | 78 (24.84) | 68 (87.18) | 10 (12.82) | ||
| Not gone into labor | 87 (27.71) | 79 (90.80) | 8 (9.20) | ||
| Any complications during or after delivery | No | 286 (91.08) | 247 (86.36) | 39 (13.64) | .035 |
| Yes | 28 (8.92) | 20 (71.43) | 8 (28.57) | ||
| Admission of baby to a hospital/NICU (n=313) | No | 190 (60.70) | 162 (85.26) | 28 (15.18) | .050 |
| Yes, for phototherapy | 85 (27.16) | 77 (90.59) | 8 (9.41) | ||
| Yes, (others) | 38 (12.14) | 28 (73.68) | 10 (26.32) | ||
| Exclusive breastfeeding (n=313) | No | 49 (15.65) | 41 (85.42) | 8 (16.67) | .282 |
| Yes | 264 (84.35) | 226 (85.61) | 38 (14.39) | ||
| Breastfeeding problem (n=313) | No | 268 (85.62) | 232 (86.57) | 36 (13.43) | .142 |
| Yes | 45 (14.38) | 35 (77.78) | 10 (22.22) | ||
| Baby crying most of the time (n=313) | No | 280 (89.56) | 241 (86.07) | 39 (13.93) | .059 |
| Yes | 33 (10.54) | 26 (78.79) | 7 (21.21) | ||
| Any congenital anomaly of the baby | No | 295 (93.95) | 253 (85.76) | 42 (14.24) | .153 |
| Yes | 19 (6.05) | 14 (73.68) | 5 (26.32) | ||
| Overall delivery experience | Good | 234 (74.52) | 207 (88.46) | 27 (11.540) | .004 |
| Bad/traumatic | 80 (25.48) | 60 (75.00) | 20 (25.00) | ||
| Number of ANC visits | <8 | 175 (55.73) | 142 (81.14) | 33 (18.86) | .030 |
| ≥8 | 139 (44.27) | 125 (89.93) | 14 (10.07) | ||
| Unfavorable obstetric history | No | 284 (90.45) | 239 (84.15) | 45 (15.85) | .180 |
| Yes | 30 (9.55) | 28 (93.33) | 2 (6.67) | ||
| Mode of delivery of the baby | Normal delivery | 169 (53.82) | 140 (82.84) | 29 (17.16) | .152 |
| Others† | 145 (46.18) | 127 (87.59) | 18 (12.41) | ||
| Gestational age (week) | <37 | 29 (9.24) | 19 (65.52) | 10 (34.48) | .003 |
| 37-40 | 256 (81.52) | 220 (85.94) | 36 (14.06) | ||
| ≥41 | 29 (9.24) | 28 (96.55) | 1 (3.45) | ||
| Birth weight of the baby (g) | <2,500 | 39 (12.42) | 28 (71.79) | 11 (28.21) | .013 |
| ≥2,500 | 275 (87.58) | 239 (86.91) | 36 (13.09) | ||
Table 4.
| EPDS questions |
n (%) |
|||||
|---|---|---|---|---|---|---|
|
Normal† |
PPD‡ |
Total |
||||
| No | Yes | No | Yes | No | Yes | |
| 1. I have been able to laugh and see the funny side of things | 263 (98.50) | 4 (1. 50) | 39 (82.98) | 8 (17.02) | 302 (96.18) | 12 (3.82) |
| 2. I have looked forward with enjoyment to things | 261 (97.75) | 6 (2.25) | 36 (76.60) | 11 (23.40) | 297 (94.59) | 17 (5.41) |
| 3. I have blamed myself unnecessarily when things went wrong | 206 (77.15) | 61 (22.85) | 13 (27.66) | 34 (72.34) | 219 (69.75) | 95 (30.25) |
| 4. I have been anxious or worried for no good reason | 200 (74.91) | 67 (25.09) | 10 (21.28) | 37 (78.72) | 210 (66.88) | 104 (33.12) |
| 5. I have felt scared or panicky for no very good reason | 221 (82.77) | 46 (17.23) | 18 (38.30) | 29 (61.70) | 239 (76.11) | 75 (23.89) |
| 6. Things have been getting on top of me | 231 (86.52) | 36 (13.48) | 17 (36.17) | 30 (63.83) | 248 (78.98) | 66 (21.02) |
| 7. I have been so unhappy that I have had difficulty sleeping | 251 (94.01) | 16 (5.99) | 16 (34.04) | 31 (65.96) | 267 (85.03) | 47 (14.97) |
| 8. I have felt sad or miserable | 255 (95.51) | 12 (4.49) | 19 (40.43) | 28 (59.57) | 274 (87.26) | 40 (12.74) |
| 9. I have been so unhappy that I have been crying | 257 (96.25) | 10 (3.75) | 25 (53.19) | 22 (46.81) | 282 (89.81) | 32 (10.19) |
| 10. The thought of harming myself has occurred to me | 264 (98.88) | 3 (1.12) | 28 (59.57) | 19 (40.43) | 292 (92.99) | 22 (7.01) |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download