Abstract
Objective
Reconstruction methods, including stent-assisted coiling, multiple telescopic stents, and flow diverters, are preferred modalities for the treatment of unruptured vertebral artery dissecting aneurysms (VADAs). We aimed to compare the clinical outcomes between two reconstructive flow diversion techniques: single flow diverter (FD) device and multiple telescopic stenting (TS).
Methods
We retrospectively reviewed the clinical data of 39 patients with unruptured VADAs. Of these, 17 patients were treated with multiple TS and 22 with a single FD device. Aneurysm characteristics and clinical outcomes were compared between the two groups.
Results
All aneurysms included in this study successfully achieved flow diversion, regardless of the treatment modality and duration. However, the mean procedure duration to complete the diversion was shorter in the FD group. Subgroup analysis in TS group showed that there were no significant clinical differences between the low-profile visualized intraluminal support and Enterprise stents, except for the mean procedure duration.
Vertebral artery dissecting aneurysms (VADAs) are a rare subtype of intracranial aneurysms. Although their incidence is low, their rupture is associated with high morbidity and mortality rates [7,14]. Patients with dissecting aneurysms may experience severe neurological deficits following hemorrhage, and secondary ischemic events upon rupture [1,4,5]. Therefore, early assertive management before rupture is necessary. With the development of diagnostic tools such as computed tomography angiography (CTA) and magnetic resonance angiography (MRA), VADAs are diagnosed more frequently before rupture. However, the natural course of these dissections remains largely unknown, and optimal treatment methods and strategies are yet to be established [7].
For the treatment of unruptured VADAs, conservative treatment with anticoagulation therapy may be considered as the first-line treatment if the degree of dissection is not severe. However, if the status worsens, including the size of the dissecting segment, changes in the shape of VADAs, and progression of related symptoms, further treatment should be considered. In these circumstances, endovascular treatment has emerged as a viable modality for VADAs [16].
Among these modalities, reconstruction methods, including stent-assisted coiling, multiple telescopic stents, flow diverters, or deconstruction methods, such as parent artery trapping, might be performed according to the characteristics of the case [10]. Because of improved maintenance of the integrity of the dominant vertebral artery or major branch involvement without adequate collateral flow, reconstructive techniques, such as flow diverters, have been preferred over deconstructive techniques, especially in cases of unruptured VADAs.
When considering flow diversion for VADAs, we prefer two methods: multiple telescopic stenting (TS) method and a single flow diverter (FD) device method. To date, several reports have identified that both multiple TS and single FD devices are effective treatment modalities for aneurysm obliteration [10,16].
However, to the best of our knowledge, there have been no studies comparing the clinical outcomes of the two modalities. Therefore, we aimed to compare the clinical outcomes between two reconstructive flow diversion techniques: single FD device and multiple TS.
We retrospectively reviewed the data of 48 patients with unruptured VADAs who were treated using endovascular reconstruction techniques at our institution between June 2015 and December 2022. All patients were initially treated medically. Patients who were had increased size on the follow-up image underwent a reconstruction procedure. Five patients treated with stent-assisted coil embolization and four patients treated with a single stent alone were excluded. Finally, 39 patients with unruptured VADAs were included; of these, 17 patients were treated with multiple TS and 22 with a single FD device.
All procedures were performed under general anesthesia using biplane angiographic equipment. An initial bolus of 50 IU/kg of heparin was injected intravenously at the beginning of the procedure. The target activated clotting time was 2–2.5 times baseline throughout the procedure.
All catheters were flushed with heparinized saline using a continuous irrigation system to prevent embolisms caused by air bubbles. The flushed saline was heparinized (1,000 IU/100 mL), and the guiding catheters and microcatheters were exposed to a continuous heparinized drip.
Standard approaches were performed through the right common femoral artery using the Seldinger technique. For the multiple TS technique, a 6-F long sheath (Flexor Shuttle, Cook Medical, Bloomington, IN, USA) or guiding catheter (Chaperone, MicroVention, Aliso Viejo, CA, USA) was used to approach the distal V2 segment. In some cases, an intermediate catheter (5-F SOFIA, MicroVention, Aliso Viejo, CA, USA) was used. A microcatheter (Headway 21, MicroVention, Aliso Viejo, CA, USA) was navigated to the distal part of the basilar artery using a microwire (Synchro-14, Stryker, Kalamazoo, MI, USA). Initially, a self-expanding stent such as Enterprise (Cerenovus, Miami, FL, USA) or low-profile visualized intraluminal support [LVIS] (MicroVention, Aliso Viejo, CA, USA), was deployed to cover the entire dissected segment. The microcatheter was then navigated to the distal part of the basilar artery again through the initially deployed stent. An overlapping stent was deployed to traverse the first stent. The same stent type was used in telescopic stenting. A triple stent was used in the Enterprise group and a double stent was used in the LVIS group.
For the single FD device technique, the guiding system was the same as that for the multiple TS technique. The microcatheter uses Phenom 27 for the Pipeline (Medtronic, Minneapolis, MN, USA) and an XT 27 flex microcatheter for the Surpass Evolve (Stryker, Portage, MI, USA) in line with the FD device. The stent or FD device sizes were determined based on the largest diameter of the parent artery and the length of the dissecting segment. In all cases, at the end of successful device deployment, control angiography was performed, and the dissecting segment margin and intra-aneurysmal contrast stagnation were carefully examined.
Dual antiplatelet premedication (aspirin 100 mg and clopidogrel 75 mg) was administered 5 days before the procedure. Dual antiplatelet therapy was maintained in all patients for 6 months and then routinely changed to antiplatelet monotherapy (aspirin 100 mg), except for one patient who developed post-procedure pontine infarction and received continuous dual antiplatelet therapy.
Diffusion-weighted imaging (DWI) magnetic resonance imaging (MRI) was routinely performed the day after the procedure, usually approximately 24 h after the procedure. DWI-positive lesions were measured manually on axial images using an institutional picture archiving and communication system (INFINTT PACS, INFINITT Healthcare, South Korea). A positive sign on follow-up DWI was defined as a lesion with a high signal intensity, regardless of the area of the brain.
Follow-up radiologic outcomes were evaluated using CTA performed at 1 month, 6 months, and 1 year after the procedure. Although catheter angiography would have provided superior imaging quality, the institution’s circumstances necessitated the use of CTA. Nonetheless, CTA’s source image provided sufficient information to analyze the outcome. During the follow-up period, the degree of diversion of the treated aneurysms, patency of the parent artery, and branch vessels covered by the stents were identified. Symptomatic complications were evaluated by diagnosing newly developed neurological symptoms after the procedure.
All statistical analyses were performed using SPSS version 26.0 (SPSS Inc., IBM Corp., Armonk, NY, USA). We performed Fisher’s exact test to analyze normal variables. The Mann–Whitney U test was performed to analyze numerical variables. Statistical differences were considered significant at p-values < 0.05.
The single FD and multiple TS groups were compared, and the baseline characteristics of the two groups are summarized in Table 1. The FD group included 14 males and 8 females with an average age of 51.0 years; the TS group included eight males and nine females with an average age of 52.06 years. All baseline characteristics of the patients in the two groups, such as hypertension, diabetes, dyslipidemia, and smoking, were not statistically significant.
The characteristics of the treated aneurysms are summarized in Table 2. All treated aneurysms were located in the V4 segment. In the FD group, 12 were on the right side and 10 were on the left; in the TS group, 11 aneurysms were on the right and 6 were on the left. The average aneurysm size was 12.63 mm and 10.96 mm for the FD and TS groups, respectively. The location (left or right) and size of the aneurysms differed between the two groups; however, the difference was not significant.
The treatment outcomes are summarized in Table 2. All aneurysms in this study successfully achieved flow diversion, regardless of the treatment modality and duration with the entire follow-up period lasting 1 year. However, the mean procedure duration to complete the diversion was notably shorter in the FD group (38.23 min) compared to in the TS group (49.47 min). In addition, the proportion of patients achieving complete diversion within 30 days was significantly higher in the FD group than in the TS group (72.7% vs. 35.3%). The differences in these factors between the two groups were statistically significant (p= 0.026). At the 6-month follow up, a significant difference was observed between the two groups (p = 0.011): compete diversion was achieved in 100% of aneurysms treated in the FD group while 70.6% in the TS group. At 1 year follow-up, both treatment modalities demonstrated comparable rates of successful diversion, with all aneurysms in both groups achieving complete diversion.
No significant differences in thromboembolic complications were identified between the two groups. Regarding DWI positivity images on Day 1 of follow-up, all lesions were identified as an embolic type, the FD group (31.8%) had a slightly higher rate than the TS group (17.6%). However, these differences did not result in significant symptomatic complications. Only one patient in the TS group showed symptoms related to thromboembolism. The DWI showed an embolic type high signal in the whole cerebellar hemisphere and the patient suffered from dizziness for several weeks.
Table 3 shows a subgroup analysis within the TS group, comparing the LVIS and Enterprise stents. Six cases were treated with the LVIS stent and 11 with the Enterprise stent. There was a significant difference in mean procedure durations. The mean duration was 39.0 min for LVIS and 55.18 min for Enterprise stent. Other factors, such as DWI positivity, symptomatic complications, and diversion at 30 days, showed no significant differences.
All aneurysms were located at V4 segment. Fig. 1 and Fig. 2 demonstrate cases of TS. Fig. 1 shows a case of triple telescopic stenting with the Enterprise stent and Fig. 2 shows a case of double telescopic stenting using LVIS stent. Fig. 3 shows a case of FD. All stents deployed at the VADAs completely covered the lesion and successful diversion of all treated aneurysms was observed in the 1-year follow-up images.
Although VADA is a less common type of intracranial aneurysm, it can be life-threatening when it ruptures because it can cause a fatal subarachnoid hemorrhage, cerebral ischemia, and mass effects associated with high morbidity and mortality [2,9,11-13]. However, the optimal treatment for unruptured VADAs remains controversial [10]. Owing to its relatively low mortality and morbidity rates, reconstructive endovascular treatment is preferred as the primary treatment modality [6,17]. Among this treatment type, FD and TS are feasible modalities for the reconstructive treatment of VADAs. In our study, these two modalities showed excellent overall outcomes; however, they exhibited different characteristics.
TS requires a more complicated procedure than FD. In the case of TS, at least two attempts of stent deployment were required to complete the procedure, and the total procedure duration was slightly longer than that of FD. Based on our data, although FD was quicker, with a mean difference of approximately 10 min, this time reduction can reduce the total procedure duration, including general anesthetic duration, and might lower the complication risk and positively impact patient outcomes.
In a subgroup analysis of the TS group, procedure duration of the Enterprise group was longer than that of the LVIS group. The Enterprise group deployed three stents, compared to two stents by the LVIS group, potentially accounting for the observed differences.
Thromboembolism is the most common complication associated with these diversion procedures, and it has been reported to occur in up to 7% of stenting procedures [3,15,18]. In our study, only one patient developed thromboembolic complication among all patients. The difference in the incidence of thromboembolic complications between the two groups was, therefore, not statistically significant. The overall incidence was 2.6%, which is less likely to be clinically significant. We believe that the low incidence of thromboembolism might be related to the short procedure time.
Theoretically, stents with a higher metal coverage rate (MCR) not only provide better flow diversion but may also provide better scaffolding for endothelialization [15]. The nominal MCR provided by the manufacturer of any FD stent is subject to change after implantation owing to factors such as the diameter of the stent relative to the parent artery and the curvature of the vessel [15]. The reported MCR of commonly used FD stents ranges from 30% to 55% [15].
We found that FD requires a shorter time to reach the final diversion state than TS. In the FD group, more than 70% of the aneurysms (72.7%) were completely diverted within only 1 month, whereas in the TS group, only 35.3% of the aneurysms were completely diverted in the same period. Our findings further support the consensus that an increased diversion rate is positively correlated with a higher MCR.
Kim et al. performed an in vitro simulation study of the hemodynamic changes in several stents and flow diverters [8]. According to these studies, MCR can change depending on the amount of compaction performed. The MCR of the double-overlapping LVIS stents ranged from 36.3% to 63.9%. However, the value of a single FD (Pipeline, Medtronic, North Ryde, Australia) varied from 26.8% to 47.8%. Since there is an overlap between the groups, we believe that it may be difficult to determine that the MCR of the Pipeline is always superior. However, the actual in vivo situation may differ and, when combined with our results, we speculate that the actual mean MCR would be higher in the FD group, which would shorten the period to the final diversion.
Nevertheless, based on our data, it is difficult to suggest that the MCR of multiple TS is inferior enough to affect the outcome compared to that of single FD. This is because all patients in our study achieved excellent results, regardless of the stent type. According to Kim et al., the average MCR of triple-overlapping Enterprise stents is only 18% [8]. However, all patients in this group, including those with double LVIS and triple Enterprise stents, achieved complete diversion. Therefore, we hypothesize that a complete diversion may occur when the minimum MCR is satisfied. The difference in MCR appears to be more closely related to the diversion period than to the complete diversion itself. However, because the number of cases in this study was too small, it was difficult to measure the specific value of minimal MCR. This should be considered in further studies.
FD appeared to achieve complete diversion slightly faster than TS; however, TS may not be an inferior choice. Each physician should choose stents according to the clinical situation of each case, and take into account that they might obtain similar results regardless of the method used.
Our study has some limitations. This was a nonrandomized, single-center, retrospective study. Therefore, the results may have been influenced by the limitations of the study design related to inherent treatment bias, and further prospective randomized controlled studies are required to confirm our results.
For the reconstructive treatment of unruptured VADAs, both the single FD and multiple TS methods showed excellent angiographic and clinical outcomes; however, single FD may be a better option because it is associated with a shorter procedure duration and faster achievement of complete flow diversion. Future prospective studies are warranted to confirm our findings.
REFERENCES
1. Ahn JY, Han IB, Kim TG, Yoon PH, Lee YJ, Lee BH, et al. Endovascular treatment of intracranial vertebral artery dissections with stent placement or stent-assisted coiling. AJNR Am J Neuroradiol. 2006; Aug. 27(7):1514–20.
2. Ahn SS, Kim BM, Suh SH, Kim DJ, Kim DI, Shin YS, et al. Spontaneous symptomatic intracranial vertebrobasilar dissection: Initial and follow-up imaging findings. Radiology. 2012; Jul. 264(1):196–202.

3. Brinjikji W, Murad MH, Lanzino G, Cloft HJ, Kallmes DF. Endovascular treatment of intracranial aneurysms with flow diverters: A meta-analysis. Stroke. 2013; Feb. 44(2):442–7.

4. Chalouhi N, Tjoumakaris S, Starke RM, Gonzalez LF, Randazzo C, Hasan D, et al. Comparison of flow diversion and coiling in large unruptured intracranial saccular aneurysms. Stroke. 2013; Aug. 44(8):2150–4.
5. Cho DY, Kim BS, Choi JH, Park YK, Shin YS. The fate of unruptured intracranial vertebrobasilar dissecting aneurysm with brain stem compression according to different treatment modalities. AJNR Am J Neuroradiol. 2019; Nov. 40(11):1924–31.

6. Drake CG, Peerless SJ. Giant fusiform intracranial aneurysms: review of 120 patients treated surgically from 1965 to 1992. J Neurosurg. 1997; Aug. 87(2):141–62.

7. Kim CH, Lee CH, Kim YH, Sung SK, Son DW, Lee SW, et al. Flow diverter devices for the treatment of unruptured vertebral artery dissecting aneurysm. J Korean Neurosurg Soc. 2021; Nov. 64(6):891–900.

8. Kim S, Yang H, Hong I, Oh JH, Kim YB. Computational study of hemodynamic changes induced by overlapping and compacting of stents and flow diverter in cerebral aneurysms. Front Neurol. 2021; Aug. 12:705841.

9. Kurata A, Ohmomo T, Miyasaka Y, Fujii K, Kan S, Kitahara T. Coil embolization for the treatment of ruptured dissecting vertebral aneurysms. AJNR Am J Neuroradiol. 2001; Jan. 22(1):11–8.
10. Lee W, Han HJ, Kim J, Park KY, Kim YB, Jang CK, et al. Flow diverter for the treatment of large (>10 mm) vertebral artery dissecting aneurysms. Acta Neurochir. 2022; May. 164(5):1247–54.

11. Mizutani T, Aruga T, Kirino T, Miki Y, Saito I, Tsuchida T. Recurrent subarachnoid hemorrhage from untreated ruptured vertebrobasilar dissecting aneurysms. Neurosurgery. 1995; May. 36(5):905–11. discussion 912-3.

12. Nakagawa K, Touho H, Morisako T, Osaka Y, Tatsuzawa K, Nakae H, et al. Long-term follow-up study of unruptured vertebral artery dissection: Clinical outcomes and serial angiographic findings. J Neurosurg. 2000; Jul. 93(1):19–25.

13. Narata AP, Yilmaz H, Schaller K, Lovblad KO, Pereira VM. Flow-diverting stent for ruptured intracranial dissecting aneurysm of vertebral artery. Neurosurgery. 2012; Apr. 70(4):982–8. discussion 988.

14. Oh HS, Bae JW, Hong CE, Kim KM, Yoo DH, Kang HS, et al. Flow diverter in unruptured intracranial vertebral artery dissecting aneurysm. Front Neurol. 2022; Jun. 13:912863.
15. Panchendrabose K, Muram S, Mitha AP. Promoting endothelialization of flow-diverting stents: A review. J Neurointerv Surg. 2021; Jan. 13(1):86–90.

16. Park KW, Park JS, Hwang SC, Im SB, Shin WH, Kim BT. Vertebral artery dissection: Natural history, clinical features and therapeutic considerations. J Korean Neurosurg Soc. 2008; Sep. 44(3):109–15.

Fig. 1.
(A) Vertebral artery dissecting aneurysm (VADA) at the right V4 segment. (B) Proximal and distal stent markers (white arrow) showed the complete deployment of the triple telescopic stenting with Enterprise stents. (C)1-year follow-up computed tomography (CT) angiography demonstrate the disappearance and complete diversion of the aneurysm.
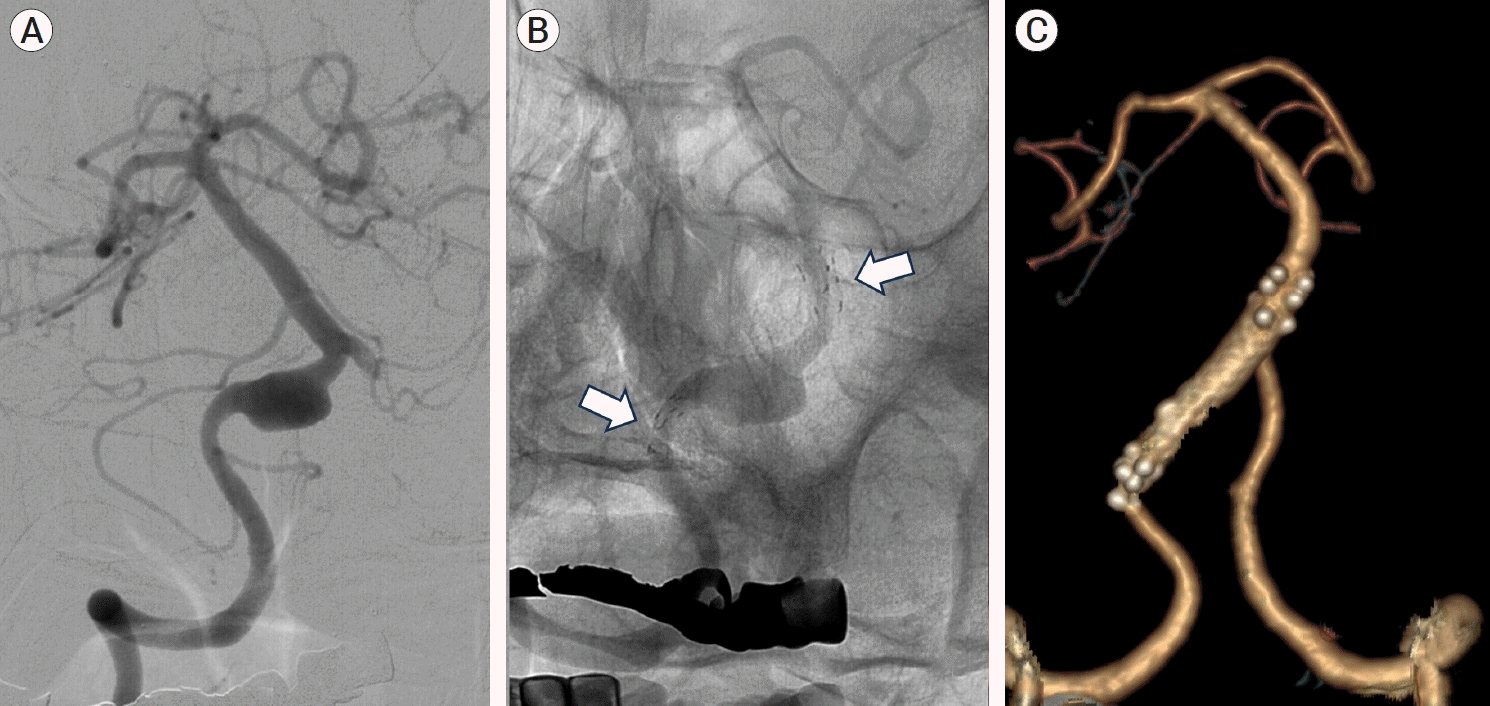
Fig. 2.
(A) Vertebral artery dissecting aneurysm (VADA) at the right V4 segment. (B) Double telescopic stenting with LVIS stent was successfully performed and stent markers showed that stents completely covered the entire aneurysm. (white arrow). (C) 1-year follow-up computed tomography (CT) angiography demonstrates the disappearance and complete diversion of the aneurysm.
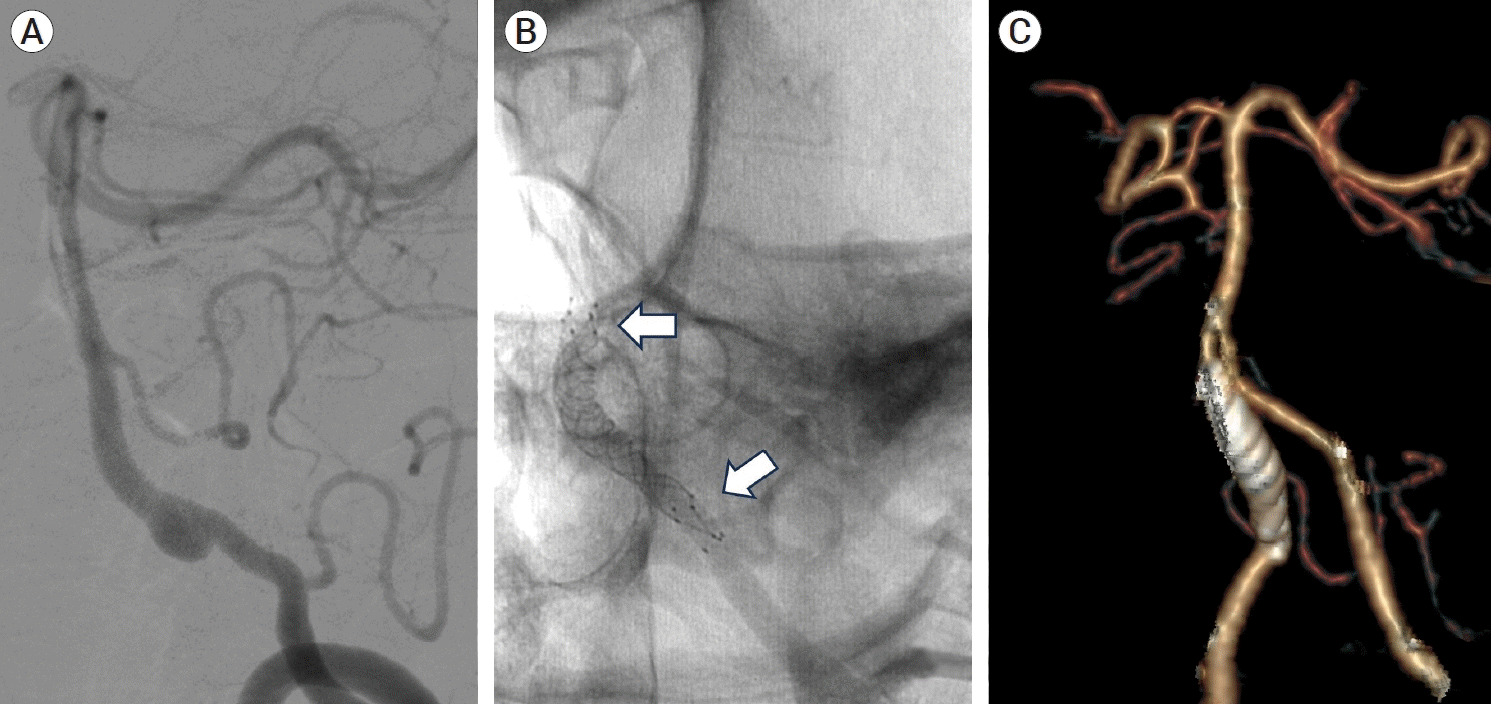
Fig. 3.
(A) Vertebral artery dissecting aneurysm (VADA) with proximal severe stenosis at the right V4 segment. (B) A single flow diverter (Pipeline) was deployed. The aneurysm was successfully covered by the diverter and improvement of the proximal stenosis was observed. (C) 1-year follow-up computed tomography (CT) angiography demonstrates the disappearance and complete diversion of the aneurysm.
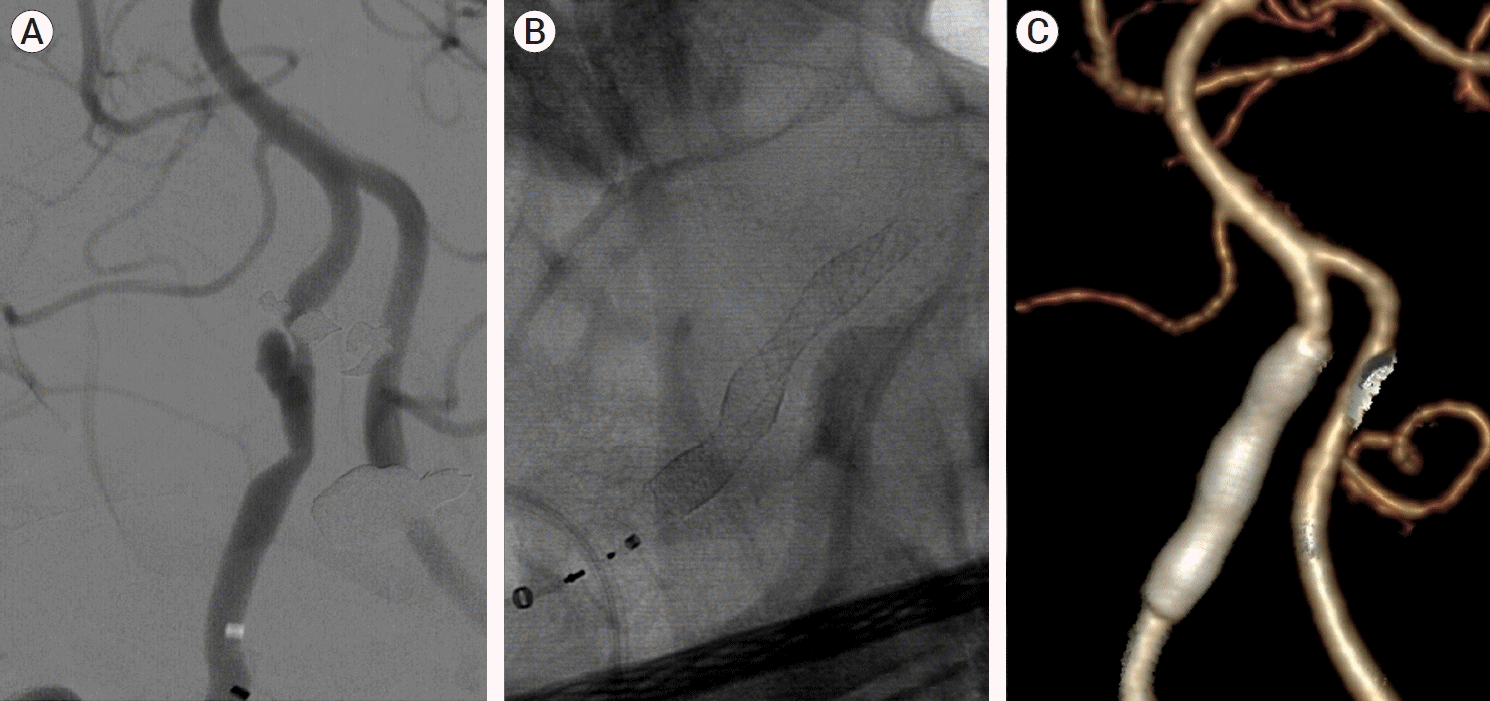
Table 1.
Demographic characteristics of patients
Table 2.
Summary of aneurysm characteristics and univariate analysis of treatment outcome




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download