Abstract
Flow diverter (FD) is increasingly used in the management of wide necked cerebral aneurysms. Despite a reported lower efficacy in middle cerebral artery (MCA) aneurysms, they are still being utilised. Microsurgery is best considered as an index treatment, but can also be a safe and effective treatment when encountering a persistent MCA aneurysm after prior FD. As there is a paucity in literature and more cases of failed FD are expected to appear, we want to add our experience to the existing literature. The microsurgical management of a persistent MCA bifurcation aneurysm, 3 years after a p48 MW HPC Flow Diverter (phenox GmbH, Bochum Germany) insertion is reported and the relevant literature discussed.
Go to : 
Flow diverters (FDs) were initially developed for proximal internal carotid artery (ICA) aneurysms. With aneurysm occlusion rates of 75-95% and low reported morbidity rates, their usage is increasing and indications are expanding [2,3,16]. They are now frequently used for more distal aneurysms such as the anterior communicating artery, anterior cerebral artery and middle cerebral artery (MCA) [7,9-11]. Optimal management of a persistent aneurysm after treatment with a FD is currently unclear.
We present a case of an MCA aneurysm for which successful adjuvant microsurgical clipping was undertaken. We did encounter some pitfalls during this procedure, due to the mechanical properties of the stent inside the parent vessel, which are worth discussing.
Go to : 
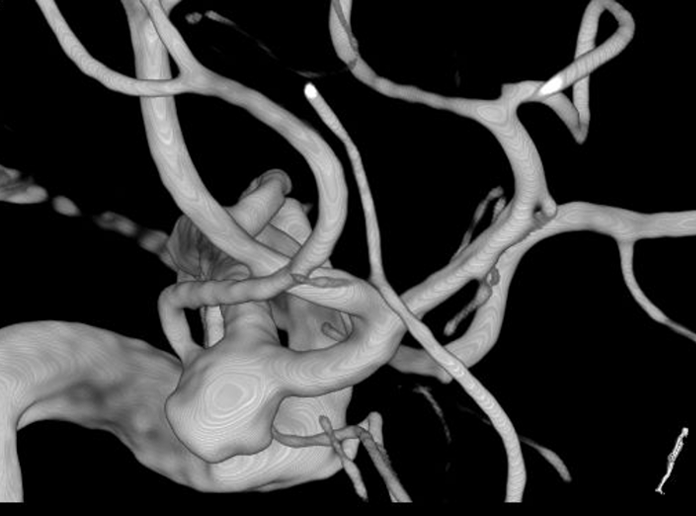
A 63-year-old female patient had an incidental finding of a right 6 mm irregular MCA bifurcation aneurysm, with the aneurysm incorporating the inferior branch (Fig. 1). There was also a left 8 mm cavernous ICA aneurysm. Cardiovascular risk factors included controlled hypertension and previous smoking.
In another centre, treatment and endovascular treatment with a FD was recommended because of the wide neck of the aneurysm. A 3×9 mm FD (p48 MW HPC phenox GmbH, Bochum Germany) was inserted without issues, she then continued on dual antiplatelets followed by aspirin alone after 6 months. The FD was placed from M1 into the frontal M2 branch, covering the neck of the aneurysm completely.
On yearly follow-up imaging, no reduction in size of the aneurysm was seen.
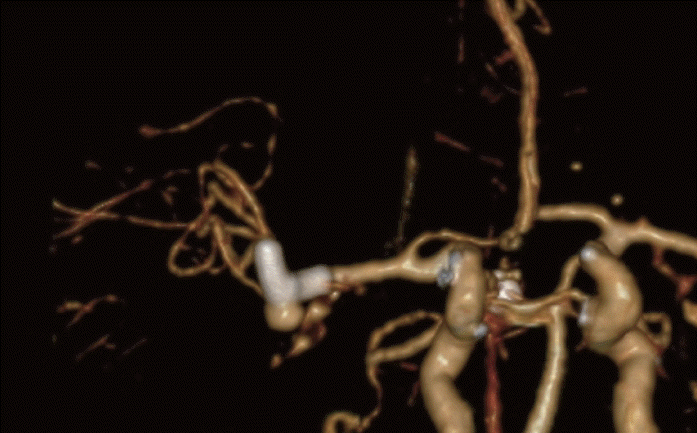
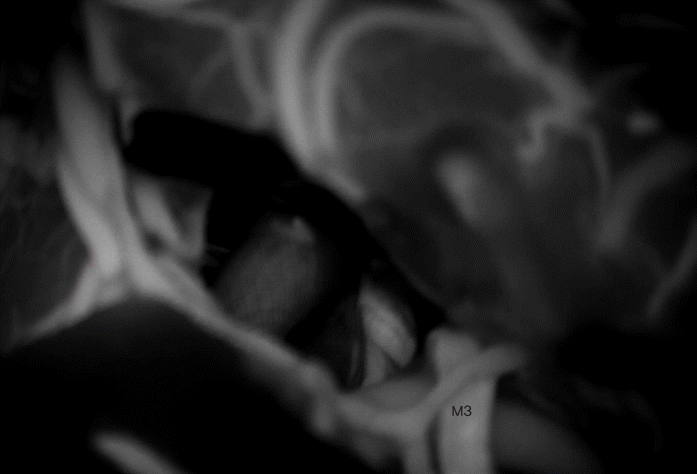
After 3 years, still no further reduction of the aneurysm was seen (Fig. 2), at which point the patient was referred to our centre for a second opinion. The decision was made to proceed to surgical clipping.
Under general anaesthesia, a right sided frontotemporal scalp incision was performed with sparing of the superficial temporal artery. A single myocutaneous flap was raised and a small pterional craniotomy was performed, followed by a focal sylvian split. Arachnoid bands could be easily cut with the 11 blade and ophthalmic blade, similar to an index operation.
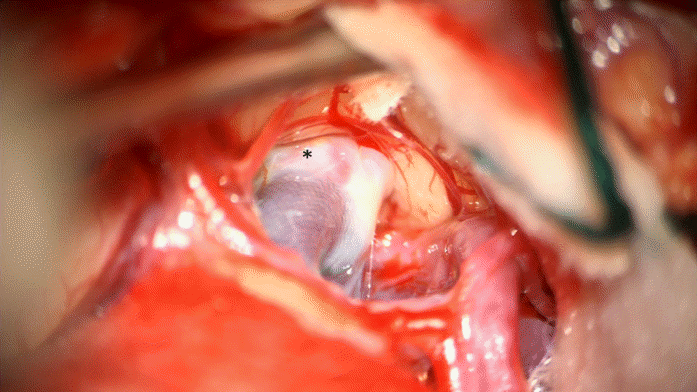
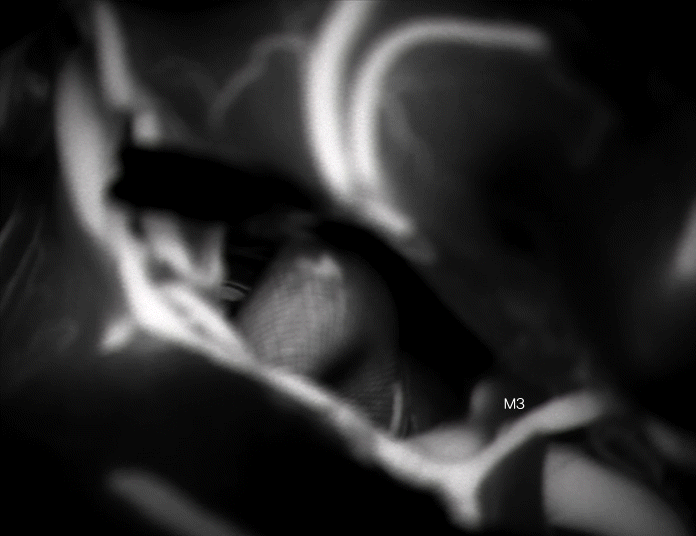
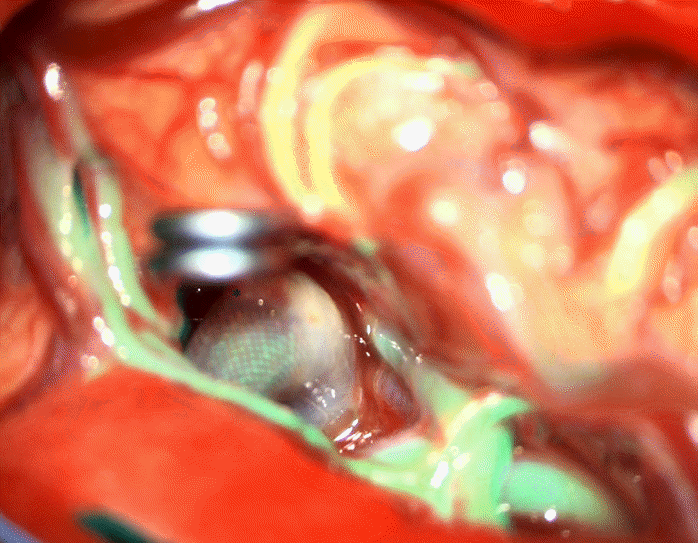
The MCA bifurcation was seen with the stent visible through the vessel wall (Fig. 3). The inferiorly projecting aneurysm was seen, with a temporal branch running over the backwall of the aneurysm (Fig. 3). No adherent cortex was encountered. The arachnoid space around the stent was intact. Exposure of the M1 segment proximal to the stent was performed in case a temporary clip needed to be placed. At first a curved 10 mm Sugita TII clip (Mizuho Co., Ltd., Tokyo, Japan) was placed; care was taken not to include the temporal branch inside the clip blades. Nonetheless indocyanine green (ICG) showed absent flow in the branch (Fig. 4), most likely due to kinking of the branch in the presence of a reduced elasticity of the parent vessel.
The curved clip was removed and replaced by a straight 10 mm clip, with repeat ICG showing normal filling of the small branch (Fig. 5). Dual image videoangiography (DIVA) or ICG overlay on Kinevo 900 (Carl Zeiss, Oberkochen, Germany) was considered a useful adjunct to classic ICG, as it allows to inspect the anatomy under white light, whilst simultaneously providing information about presence or absence of blood flow (Fig. 6). A small dog ear remnant was clipped with a second straight mini-clip.
No retractors or temporary clips were used during the procedure.
The postoperative course was unremarkable. Postop computed tomography angiography (CTA) showed a complete exclusion of the aneurysm. The patient was discharged home postop day 5 and after 6 weeks the patient had resumed her previous activities.
Go to : 
FD is one of the many tools that has been added to the endovascular armamentarium. Low morbidity, easeof-use and high efficacy has contributed to its increasing usage and broadening indications [2,3,16]. Although its role is well established in proximal ICA aneurysms, use in distal locations such as the MCA remains controversial [19].
A study from 2016 assessed the use of FD in 29 MCA aneurysms, including M1, bifurcation and distal MCA aneurysms, as well as saccular, fusiform and previously treated aneurysms. Subgroup analysis concluded FD was an indispensable tool for the management of fusiform and dissecting MCA aneurysms, but that FDs should not be considered as the primary treatment for MCA bifurcation aneurysms [20].
A 2017 systematic review and meta-analysis of 244 MCA aneurysms treated with FD showed an 80% overall rate of complete/near-complete occlusion, with an overall complication rate of 20% resulting in permanent neurologic deficits in approximately 10% of patients and treatment-related mortality in about 2% [8].
A more recent multicenter retrospective study reevaluated 54 MCA bifurcation aneurysms that underwent flow diversion [12]. The cohort was mixed with four (7.4%) ruptured aneurysms and 14 (25.9%) of aneurysms already undergoing either open surgery or coiling prior to flow diversion. 16.7% (9/54) of the patients suffered a thromboembolic complication, and from the 45 aneurysms with available follow-up data, 20% did not have adequate occlusion at the median follow-up time of 12 months. They concluded FD is a viable option for complex MCA aneurysms, however outcomes are inferior and are associated with a higher rate of complications when compared to clipping [12].
More favourable results were published by Piano et al. who reported a complete occlusion rate of 91% and overall morbidity of 8.5%, but 2.1% mortality. There was an occlusion rate of 81% in the saccular subtype of aneurysms. In the remaining aneurysm subtypes, 100% aneurysm occlusion was achieved [18].
Despite these results, FDs are still used and offered as a primary treatment in non-complex saccular MCA aneurysms. A recent pilot safety study used the p48 MW HPC stent, the same as in our patient, but in combination with prasugrel monotherapy. 21 patients with 27 unruptured aneurysms were included, of which 24 MCA aneurysms (16 MCA bifurcation, 6 M1, 2 M2). 20% ischemic complications were registered, at 2 years follow-up there was an overall occlusion rate of 74%. Important to note, is that only 50% (10/20) of the MCA aneurysms achieved occlusion [10,11].
Microsurgery on the other hand is still superior in terms of complications and occlusion rate. A recent meta-analysis and systematic review comparing microsurgical clipping to new endovascular techniques, showed a 95.7% occlusion rate for clipped MCA aneurysms with a treatment related complication rate of 2.9% versus 78.1% occlusion rate and 5.6% complication rate for endovascular interventions (flow diverters 84.3% and 7.3%; flow disruptors 78% and 14%; stent-assisted coiling 82% and 5.4%; p-conus 50% and 3.5%) [19]. However these results are mainly based on retrospective studies; an randomized controlled trial (RCT) comparing surgery with endovascular management for unruptured MCA aneurysms is yet to be performed.
For patients who refuse surgery or are not fit for surgery, other endovascular treatment might be considered.
The rationale for FD in aneurysms is twofold: firstly, as the name implies, it diverts flow away from the aneurysm with stagnation of flow inside the aneurysm lumen making it prone to thrombosis. Secondly, the FD serves as a scaffold for new endothelialization that remodels the parent artery and seals the communication with the aneurysm in a delayed fashion [5]. The reasons for failed FD are commonly technical, with a stent not completely across the neck seen in up to 5%, or a stent that is either under-or oversized. Delayed stent migration after deployment has also been reported. We encountered a 61-year-old patient with a persistent right M1 aneurysm, treated elsewhere with FD, however follow-up digital substraction angiography (DSA) showed the stent was sitting too proximal, the patient unfortunately refused further treatment.
In this case report, the FD was inserted correctly, but aneurysm size did not reduce after 3 years. As discussed above, particularly MCA bifurcation aneurysms respond poorly to FD insertion [8-12]. This is most likely due to the presence of a branch vessel coming out of the aneurysm [5]. This was also seen pre- and intraoperatively in our case with the M2 temporal branch emerging from the aneurysm (Fig. 3).
A meta-analysis of FD for unruptured distal anterior circulation aneurysms found MCA location to be a predictor for aneurysm non-occlusion (versus anterior communication artery or distal anterior cerebral artery, OR 0.5, P+.03) [9]. There are also different occlusion rates depending on the type of FD, with Pipeline Embolization Device (PED) offering the best reported occlusion rates [9].
The best strategy for treatment of residual or recurrent aneurysms after Flow Diversion is unclear, as literature on this subject is sparse [14,15]. Prevention is better than cure; choosing the correct index treatment cannot be overemphasized. If a FD is chosen and a persistent aneurysm is encountered, coiling is not possible because the braids of a FD stent do not allow a microcatheter to cross them [5]. So either further conservative treatment, microsurgery or repeat endovascular therapy with another FD or stent insertion can be considered.
Unlike clipping or coiling, aneurysm occlusion after FD is a gradual process, this is reflected in the increasing occlusion rates when assessing patients after 1, 6, 12 or 24 months [10,11]. In our case there was no volume reduction after 3 years. It was considered unlikely a sudden aneurysm occlusion or reduction would occur.
Repeat endovascular treatment has been performed and advocated. One multicenter retrospective study included 11 patients that underwent retreatment with FD for 12 aneurysms initially treated with FD. These were ICA and posterior circulation aneurysms however, and they reported a complete or near-complete aneurysm occlusion rate of 91.7%, and a 16.6% technical complication rate [14].
Another study included 29 aneurysms: 16 ICA, 2 MCA and 11 posterior circulation aneurysms. A total of 42 retreatments using the PED were performed [15]. Angiographic improvement was observed in 45% of aneurysms and complete occlusion in 26% of aneurysms following retreatments. Clinical complications occurred in 10% (4/42) of PED retreatments, 7% (3/42) ipsilateral ischemic strokes and 2% (1/42) subarachnoid hemorrhage. One ischemic stroke and one subarachnoid hemorrhage occurred after retreatment of aneurysms in the anterior circulation, while two ischemic strokes occurred following retreatment of aneurysms in the posterior circulation. The subarachnoid hemorrhage was the result of balloon angioplasty following PED deployment and resulted in patient death. Their reported ischemic risk was nearly twice that reported in the IntrePED study of 906 (4.5%) aneurysms treated with PED [7].
Only a few reports in literature appear regarding the surgical nuances of treatment after previous FD [1,13,17].
The exposure was not much different from an index surgery, we did not encounter thickened arachnoid or adherent brain cortex. The only difficulties encountered were related to the reduced elasticity of the artery: the presence of an intraluminal FD creates an increased stiffness, which makes it more difficult to mobilize the parent vessel and inspect the neck of the aneurysm. A 30-degree angled endoscope has been recommended to aid with this, however this was unnecessary in our case [17].
The reduced compliance could make the neck of the aneurysm more prone to rupture, or, as was seen in our case, could kink one of the branches or perforators. Fortunately this was picked up with ICG and corrected by clip replacement. This highlights the importance of intraoperative ICG with confirmation that FDs are ICG translucent.
Although we try to avoid the use of temporary clips, in certain situations it is unavoidable. In vitro studies have shown that placing a temporary clip on a PED-stent will irreversibly damage the stent.4) Furthermore flow arrest did not occur with the first clip application in these cases. To overcome this potential issue, we exposed more of the proximal MCA in case intraoperative rupture would occur.
Other surgical strategies described include clip reconstruction with removal of the device, trapping with or without bypass, proximal occlusion and wrapping [17]. More data is needed to define the optimal strategy when dealing with persistent aneurysms after previous flow diversion.
Go to : 
Notes
Disclosure
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Go to : 
REFERENCES
1. Al-Schameri AR, Lunzer M, Daller C, Kral M, Killer M. Middle cerebral artery aneurysm surgery after stent misplacement: A case report. Interv Neuroradiol. 2016; Feb. 22(1):49–52.

2. Austerman RJ, Sadrameli SS, Guerrero JR, Wong M, Diaz O, Klucznik R, et al. Is flow diversion the death of simple coiling or stent-assisted coiling? A single-center experience. Curr Neurovasc Res. 2020; 17(5):754–59.

3. Becske T, Potts MB, Shapiro M, Kallmes DF, Brinjikji W, Saatci I, et al. Pipeline for uncoilable or failed aneurysms: 3-year follow-up results. J Neurosurg. 2017; 127(1):81–8.
4. Bell RS, Bank WO, Armonda RA, Vo AH, Kerber CW. Can a self-expanding aneurysm stent be clipped? Emergency proximal control options for the vascular neurosurgeon. Neurosurgery. 2011; Apr. 68(4):1056–62.

5. Bonney PA, Connor M, Fujii T, Singh P, Koch MJ, Stapleton CJ, et al. Failure of flow diverter therapy: Predictors and management strategies. Neurosurgery. 2020; Jan. 86(Suppl 1):S64–73.

6. Bowers CA, Taussky P, Park MS, Neil JA, Couldwell WT. Rescue microsurgery with bypass and stent removal following Pipeline treatment of a giant internal carotid artery terminus aneurysm. Acta Neurochir (Wien). 2015; Dec. 157(12):2071–5.

7. Brinjikji W, Lanzino G, Cloft HJ, Siddiqui AH, Boccardi E, Cekirge S, et al. Risk factors for ischemic complications following pipeline embolization device treatment of intracranial aneurysms: Results from the IntrePED Study. AJNR Am J Neuroradiol. 2016; Sep. 37(9):1673–8.

8. Cagnazzo F, Mantilla D, Lefevre PH, Dargazanli C, Gascou G, Costalat V. Treatment of middle cerebral artery aneurysms with flow-diverter stents: A systematic review and meta-analysis. AJNR Am J Neuroradiol. 2017; Dec. 38(12):2289–94.
9. Cagnazzo F, Perrini P, Dargazanli C, Lefevre PH, Gascou G, Morganti R, et al. Treatment of unruptured distal anterior circulation aneurysms with flow-diverter stents: A meta-analysis. AJNR Am J Neuroradiol. 2019; Apr. 40(4):687–93.

10. de Castro-Afonso LH, Machado JP, Nakiri GS, Abud TG, Monsignore LM, Freitas RK, et al. Two year follow-up of distal unruptured intracranial aneurysms treated with a surface modified flow diverter under prasugrel monotherapy. J Neurointerv Surg. 2023; Jul. jnis-2023-020397.

11. de Castro-Afonso LH, Nakiri GS, Abud TG, Monsignore LM, Freitas RK, de Oliveira RS, et al. Treatment of distal unruptured intracranial aneurysms using a surface-modified flow diverter under prasugrel monotherapy: A pilot safety trial. J Neurointerv Surg. 2021; Jul. 13(7):647–51.

12. Diestro JDB, Adeeb N, Dibas M, Boisseau W, Harker P, Brinjikji W, et al. Flow diversion for middle cerebral artery aneurysms: An international cohort study. Neurosurgery. 2021; Nov. 89(6):1112–21.

13. Ding D, Liu KC. Microsurgical extraction of a malfunctioned pipeline embolization device following complete deployment. J Cerebrovasc Endovasc Neurosurg. 2013; Sep. 15(3):241–5.
14. Goertz L, Hesse N, Liebig T, Ahmad W, Abdullayev N, Krischek B, et al. Retreatment strategies for recurrent and residual aneurysms after treatment with flow-diverter devices. Neuroradiology. 2020; Aug. 62(8):1019–28.

15. Lauzier DC, Cler SJ, Chatterjee AR, Osbun JW, Moran CJ, Kansagra AP. Retreatment of previously flow diverted intracranial aneurysms with the pipeline embolization device. Interv Neuroradiol. 2023; Dec. 29(6):710–4.

16. Limbucci N, Leone G, Renieri L, Nappini S, Cagnazzo F, Laiso A, et al. Expanding indications for flow diverters: Distal aneurysms, bifurcation aneurysms, small aneurysms, previously coiled aneurysms and clipped aneurysms, and carotid cavernous fistulas. Neurosurgery. 2020; Jan. 86(Suppl 1):S85–94.

17. Mbabuike N, Shakur SF, Gassie K, Srinivasan V, Mascitelli J, Abla A, et al. Microsurgical management of intracranial aneurysms after failed flow diversion. World Neurosurg. 2020; Feb. 134:e16–28.
18. Piano M, Lozupone E, Milonia L, Pero G, Cervo A, Macera A, et al. Flow diverter devices in the treatment of complex middle cerebral artery aneurysms when surgical and endovascular treatments are challenging. J Stroke Cerebrovasc Dis. 2022; Oct. 31(12):106760.
19. Toccaceli G, Diana F, Cagnazzo F, Cannizzaro D, Lanzino G, Barbagallo GMV, et al. Microsurgical clipping compared with new and most advanced endovascular techniques in the treatment of unruptured middle cerebral artery aneurysms: A meta-analysis in the modern era. World Neurosurg. 2020; May. 137:451–64.

Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download