Abstract
Partial trisomy of the long arm of chromosome 17 (17q) is a rare but clinically recognized syndrome that involves facial dysmorphisms, skeletal abnormalities, and global developmental delay, as well as various reports of cardiovascular, renal, and central nervous system abnormalities. This report presents a novel neuroradiologic finding of diffuse enlarged, tortuous cortical veins with physiological antegrade flow in a child with a microduplication of the distal end of 17q. To our knowledge, this finding has not been described previously. Although the exact cause for the cortical vascular anomaly is currently unknown, this duplicated region contains genes of interest for future studies that focus on normal and abnormal angiogenesis
Partial 17q trisomy is a rare phenomenon that can involve various segments of the long arm of chromosome 17 resulting in a distinct clinical syndrome. The first reports of partial 17q trisomy were in 1978 and it was recognized as a distinct syndrome in 1984 [4]. The majority of these cases in the literature involve translocations and monosomies of other chromosomes. However, there are reported instances of de novo pure microduplications which only involve the terminal end of chromosome 17q [3,8]. Despite the involvement of other chromosomes and the varied regions of chromosome 17q involved, partial trisomy of 17q typically presents as a distinct clinical syndrome. The characteristic features include facial dysmorphisms, skeletal abnormalities, and global developmental delay. In addition, there are often abnormalities of the cardiovascular system, renal system, and central nervous system, but the types of abnormalities are quite varied [2,4,11]. The central nervous system (CNS) abnormalities that have been reported include cerebral edema, cerebral volume loss, lissencephaly, Dandy-Walker malformation, holoprosencephaly, etc [2,4,6,7].
We describe the unique neuroimaging findings of an 8-year-old girl with a pure microduplication of 17q25.1 -q25.3. MR venography and digital subtraction angiography showed peculiar, widespread cortical vein abnormalities while the cerebral arteries, capillaries, and dural venous sinuses all appeared normal. To our knowledge, this finding has never been described but may represent one of the varied CNS abnormalities seen in many of the patients with partial trisomy of distal 17q.
A 12-month-old female of Mexican descent with unremarkable prenatal course and term birth initially presented for a routine well child visit and was found to have gross motor delay but was otherwise healthy. Over time, motor delays persisted, and she was noted to have several mild dysmorphologies including a low posterior hairline, mild macrocephaly, a broad neck, borderline hypertelorism, posteriorly rotated ears, slanting backwards of the front teeth, a high arched palate, a broad chest with widely spaced nipples, flat feet, mild hypotonia, arachnodactyly, and a wide based gait with balancing correction when walking. Of note, she did not have any genitourinary abnormalities or cardiac murmurs noted on physical examination, nor did she have any neurologic symptoms such as seizures, headaches, or somnolence.
Given dysmorphism and psychomotor delay, she was referred for genetic testing which revealed a microduplication of 17q25.1-q25.3. The duplicated region was 2.8 Mb in length and contained 61 genes. Her parents were both tested but neither contained a similar duplication, so her chromosomal abnormality was determined to be a de novo microduplication that was likely clinically significant. She began to follow with a genetics clinic and underwent formal neuropsychological evaluation. Neuropsychological testing revealed global developmental delay and highlighted a significant weakness in speech and language skills. She did not have any symptoms of elevated intracranial pressure, such as headaches, somnolence, or seizure. Given reports in the literature of CNS and genitourinary anomalies, an echocardiogram, renal ultrasound, and CT abdomen and pelvis were obtained and were normal.
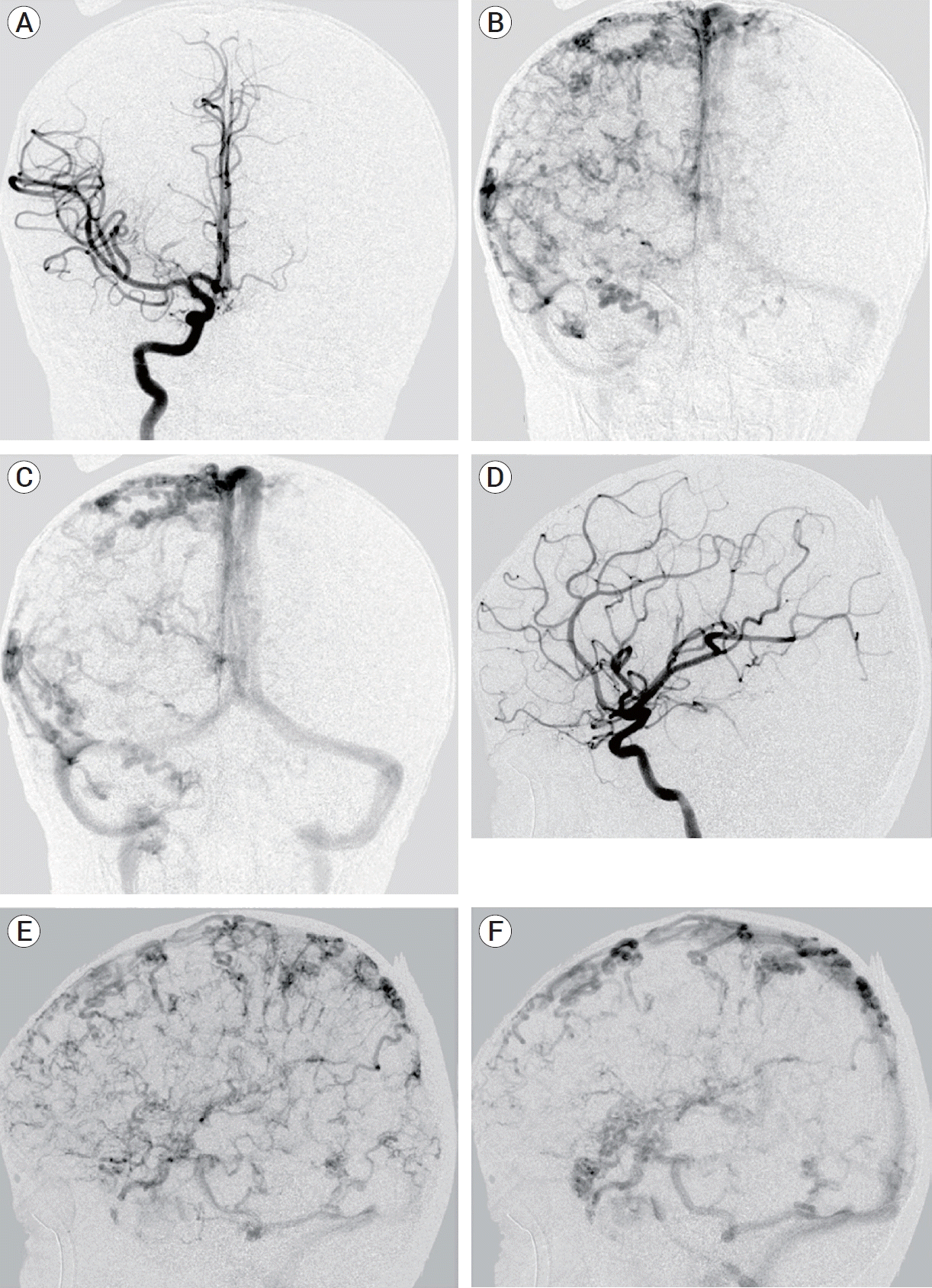
A brain magnetic resonance imaging (MRI) and magnetic resonance angiography (MRA) obtained at the age of 5 revealed a normal brain and cerebral arteries, but showed numerous enlarged, tortuous cortical veins draining into normal appearing dural venous sinuses, and supratentorial and infratentorial veins were affected. (Fig. 1) As a result of the cortical vein abnormalities, she was referred to neurosurgery for further evaluation. Cerebral angiography showed similar venous anomalies with no early filling of the cortical veins, arteriovenous fistulas, or arteriovenous malformations. (Fig. 2) A repeat surveillance brain magnetic resonance venography (MRV) obtained at age 8 showed more pronounced tortuous, distended veins overlying the cerebral cortices and internal cerebral veins with patent major intracranial dural sinuses and deep cerebral veins.
At the time of this writing, she is progressing well with intensive speech and occupational therapy, and she developed menarche at the age of 11. However, she has developed obesity and hepatic steatosis without evidence of diabetes or other endocrine dysfunction. These may be unrelated to her genetic condition given a significant family history for both conditions.
We describe a case of previously unreported diffuse engorged, tortuous cortical veins without signs of arteriovenous shunting in a girl with developmental delay, dysmorphism, and partial 17q microduplication. Partial trisomy of 17q is a rare syndrome resulting from chromosomal aberrations that can occur de novo or as the result of an inherited parental chromosomal abnormality [4]. Patients with this syndrome often have developmental delay, intellectual disability, and facial and body dysmorphism [2,4]. Many also have cardiovascular abnormalities including total anomalous pulmonary venous return, coarctation of the aorta, and atrial or ventricular septal defects [7]. Genitourinary malformations, such as cryptorchidism, and central nervous system abnormalities, such as cerebral atrophy, cerebral edema, DandyWalker malformation, holoprosencephaly, microlissencephaly, and corpus callosum abnormalities have also been reported [2,4,6,7]. Unlike our patient, the majority of 17q duplications extend to the terminus of the chromosome because of an unbalanced translocation with another chromosome, as opposed to a pure trisomy of 17q. Further, only a few authors have reported de novo microduplications with smaller affected regions [2,3,12]. The patient described above has some of the features seen in 17q duplication syndrome, but the exact microduplication and neuroimaging findings described in our patient has not been previously documented, to our knowledge.
The classically described venous drainage pattern in an adult is consists of superficial and deep systems. The superficial cortical veins drain the outer cortex in a centrifugal direction and continue toward their dural sinuses [5]. Dilated cortical veins can develop because of increased pressure in the venous system, often due to dural arteriovenous fistula or dural venous sinus thrombosis. Although rare, dural arteriovenous fistulas can present in infants due to thrombosed dural sinuses or other venous obstruction, leading to tortuous, engorged veins. These fistulas are more commonly seen in older adults as the result of dural sinus thrombosis secondary to trauma or inflammation. The engorged, tortuous venous pattern has been described as a pseudophlebitic pattern and reflects venous congestion [13]. In our patient, angiography revealed antegrade venous drainage without either early or late filling. (Fig. 2) No arteriovenous fistula or arteriovenous malformation was present by catheter angiography, MRA or MRV. Although the cortical veins in our patient have a similar appearance as the pseudophlebetic pattern described with dural arteriovenous fistulas, this explanation is less likely given the lack of fistulas on imaging, the lack of dural sinus stenosis or thrombosis on imaging, and the diffuse nature of the anomaly involving all cortical veins.
While the cause of this novel vascular phenotype is not known, upregulation of one or more of the 61 genes in the duplicated region in this patient likely causes or contributes to this disorder. The region contains SEC14L1, a gene that codes for a Sec14-like phosphatidylinositol lipid transfer protein. Little functional data is available for SEC14L1, but its paralog SEC14L2 functions as a key mediator of vasculogenesis by promoting VEGFR2 activation by phosphorylating a specific tyrosine residue [1]. SEC14L1 is likely to have an important role in vasculogenesis as well as it is highly expressed in vascular endothelial cells based on data from The Human Protein Atlas [10]. Further, increased copy number (and presumed upregulation) of SEC14L1 has been associated with lymphovascular invasion and high histological grade in breast cancer [9].
To our knowledge, this is the first time this type of abnormality has been described although it is possible that other cases may have gone undetected due to lack of dedicated imaging. This patient was not experiencing any neurologic symptoms at the time of initial imaging and remains free of any neurologic complications. The natural history of these abnormal veins is uncertain, and this patient will continue to undergo surveillance imaging and follow up over time.
We report a unique cortical venous abnormality that, to our knowledge, has not been previously described in a patient with partial duplication of 17q. Given the uncertainty of its natural course or related complications, this may be an indication to consider brain MRI/MRV in patients with similar chromosomal abnormalities. In addition, more research into SEC14L1 should be considered as they may play a role in the abnormal phenotype seen in this patient in the setting of a modest gain of function mutation.
REFERENCES
1. Gong B, Li Z, Xiao W, Li G, Ding S, Meng A, et al. Sec14l3 potentiates VEGFR2 signaling to regulate zebrafish vasculogenesis. Nat Commun. 2019; Apr. 10(1):1616.

2. Hackmann K, Stadler A, Schallner J, Franke K, Gerlach EM, Schrock E, et al. Severe intellectual disability, West syndrome, Dandy-Walker malformation, and syndactyly in a patient with partial tetrasomy 17q25.3. Am J Med Genet Part A. 2013; Dec. 161A(12):3144–9.

3. Kelly BD, Becker K, Kermode V, Stallings RL, Murphy RP, Green AJ, et al. Dysmorphic features and learning disability in an adult male with pure partial trisomy 17q24-q25 due to a terminal duplication. Am J Med Genet. 2002; Oct. 112(2):217–20.
4. Naccache NF, Vianna-Morgante AM, Richieri-Costa A. Brief clinical report: Duplication of distal 17q: Report of an observation. Am J Med Genet. 1984; Mar. 17(3):633–9.
5. Pearl M, Gregg L, Gandhi D. Cerebral venous development in relation to developmental venous anomalies and vein of galen aneurysmal malformations. Semin Ultrasound CT MR. 2011; Jun. 32(3):252–63.

6. Poulton CJ, Schot R, Seufert K, Lequin MH, Accogli A, Annunzio GD, et al. Severe presentation of WDR62 mutation: Is there a role for modifying genetic factors? Am J Med Genet Part A. 2014; Sep. 164A(9):2161–71.
7. Probst FJ, James RA, Burrage LC, Rosenfeld JA, Bohan TP, Ward Melver CH, et al. De novo deletions and duplications of 17q25.3 cause susceptibility to cardiovascular malformations. Orphanet J Rare Dis. 2015; Jun. 10(1):1–9.

8. Shimizu T, Ikeuchi T, Shinohara T, Ohba S, Miyaguchi H, Akiyama T, et al. Distal trisomy of chromosome 17q due to inverted tandem duplication. Clin Genet. 1988; Apr. 33(4):311–4.
9. Sonbul SN, Aleskandarany MA, Kurozumi S, Joseph C, Toss MS, Diez-Rodriguez M, et al. Saccharomyces cerevisiae-like 1 (SEC14L1) is a prognostic factor in breast cancer associated with lymphovascular invasion. Mod Pathol. 2018; Nov. 31(11):1675–82.

10. Uhlén M, Fagerberg L, Hallström BM, Lindskog C, Oksvold P, Mardinoglu A, et al. Proteomics. Tissue-based map of the human proteome. Science. 2015; Jan. 347(6220):1260419.
11. Upadia J, Philips JB 3rd, Robin NH, Lose EJ, Mikhail FM. A case report of chromosome 17q22-qter trisomy with distinct clinical presentation and review of the literature. Clin Case Rep. 2018; Feb. 6(4):612–6.

12. Vidović V, Maksimović N, Damnjanović T, Jekić B, Milovac I, Grk M, et al. Findings from ACGH in patient with psychomotor delay-case report. Genetics & Applications,. 2019; 3(3):38–41.

13. Willinsky RA, Goyal M, TerBrugge K, Montanera W. Tortuous, engorged pial veins in intracranial dural arteriovenous fistulas: Correlations with presentation, location, and MR findings in 122 patients. AJNR Am J Neuroradiol. 1999; JunJul. 20(6):1031–6.
Fig. 1.
(A) Axial T2-weighted MRI obtained at the age of 5 shows numerous tortuous cortical veins (white arrows). (B) Coronal T2-weighted MRI shows numerous tortuous cortical veins (white arrows). (C) Full-field coronal maximum intensity projection of contrast- enhanced Time-of-flight MRA shows tortuous cortical veins (white arrows) and normal appearing arterials and major dural venous sinuses. MRI, magnetic resonance image; MRA, magnetic resonance angiography
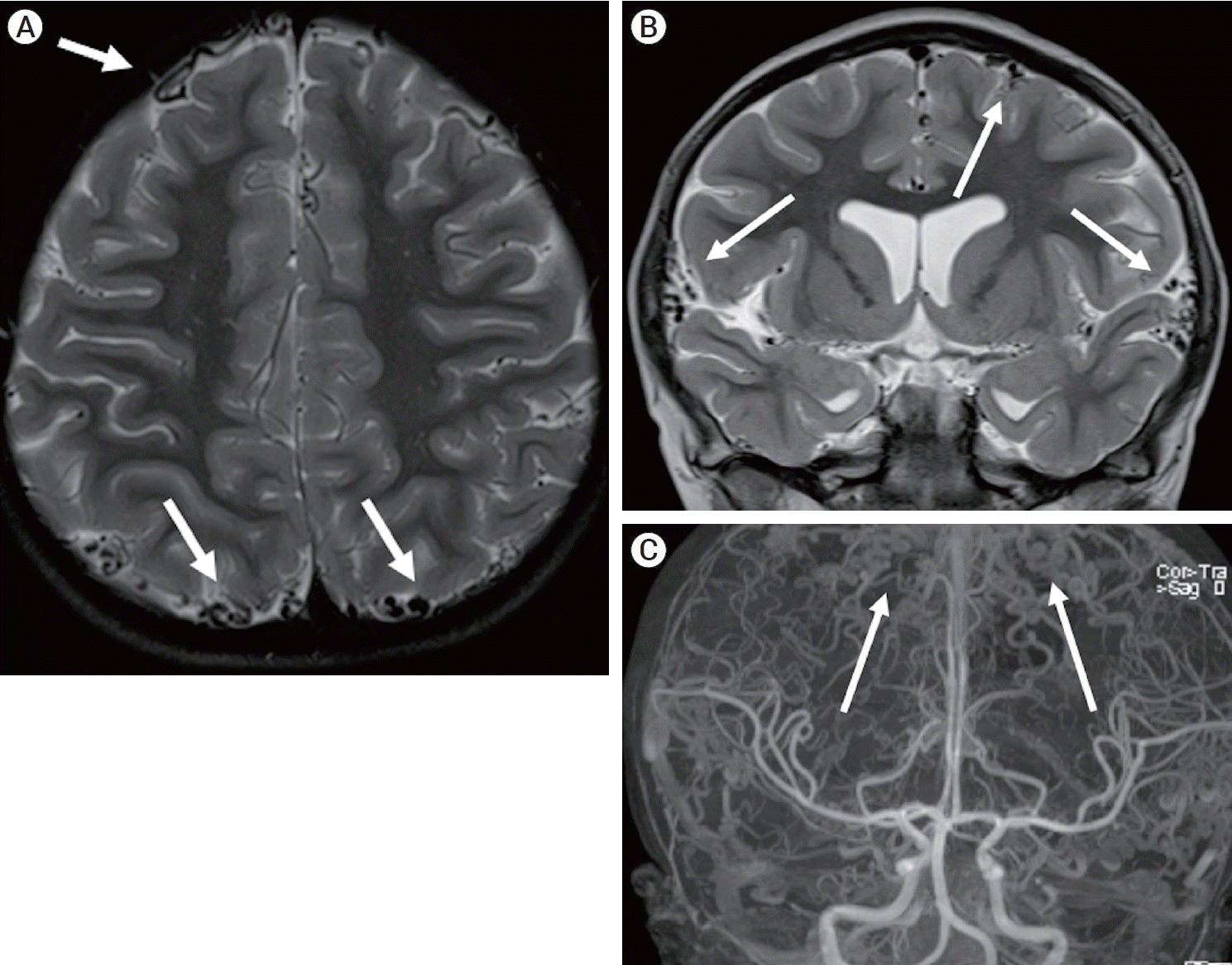
Fig. 2.
(A) Anteroposterior (AP) catheter angiogram at arterial phase demonstrate normal arterial anatomy without arteriovenous shunting. (B) AP catheter angiogram at early venous phase demonstrate diffuse tortuous cortical veins. (C) AP catheter angiogram at late venous phase demonstrate tortuous cortical veins draining into normal appearing major venous sinuses. (D) Lateral catheter angiogram at arterial phase demonstrate normal arterial anatomy without arteriovenous shunting. (E) Lateral catheter angiogram at early venous phase demonstrate diffuse tortuous cortical veins. (F) Lateral catheter angiogram at late venous phase demonstrate tortuous cortical veins draining into normal appearing major venous sinuses.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download