Abstract
Background: Dengue virus (DENV) is transmitted by mosquitoes and is becoming a global threat owing to an increase in the number of cases and mortality, especially in low- and middle-income tropical countries. Rapid and easy-to-use diagnostic tests are required to differentiate dengue fever from other febrile illnesses. Methods: We evaluated the clinical performance of the ImmuneMed Dengue NS1 Ag Rapid I test (ImmuneMed, Inc.) using positive and negative sera collected from patients with confirmed DENV infection and healthy individuals, respectively. The AccuPower® ZIKV (DENV, CHIKV) multiplex real-time reverse transcription polymerase chain reaction (RT-PCR) assay (Bioneer) was used as the reference standard to confirm DENV infection. Results: One hundred DENV-positive and 161 DENV-negative samples were evaluated. Overall, the sensitivity and specificity were 100% (95% confidence interval [CI], 96%–100%) and 100% (95% CI, 98%–100%), respectively. The sensitivity and specificity were 100% for both DENV-1 and DENV- 2. The sensitivity was the same (100%) for sera collected < 3 days and ≥ 3 days from symptom onset. The performance of the ImmuneMed Dengue NS1 Ag Rapid I and realtime RT-PCR tests showed strong overall agreement. Conclusion: The ImmuneMed Dengue NS1 Ag Rapid I test was highly specific for DENV and as sensitive as RT-PCR. These findings suggest that the ImmuneMed Dengue NS1 Ag Rapid I test may be a useful point-of-care test for dengue fever.
Go to : 
Dengue fever is a mosquito-borne disease caused by dengue virus (DENV). DENV comprises four antigenically related, but distinct serotypes: DENV-1, DENV-2, DENV-3, and DENV-4 [1]. A newly discovered fifth serotype (DENV-5), closely related to DENV-4, was isolated in Malaysia in 2007 [2]. Genetic analyses revealed that the new serotype (DENV-5) is phylogenetically distinct from the other four serotypes [2,3]. Mild-to-severe infections can occur with one or more of the four serotypes. Infection with any of the four DENV serotypes provides lifelong immunity to that particular serotype, but only temporary immunity against other serotypes [4]. Epidemiological studies have shown that subsequent infections with different DENV serotypes increase the risk of developing severe dengue hemorrhagic fever and dengue shock syndrome [5-9].
The clinical manifestations of DENV infection depend on factors such as the DENV serotype and the immune status, age, and underlying medical conditions of the host [10]. Several studies have described the clinical characteristics of different dengue serotypes. DENV-1 and DENV-4 are associated with milder illnesses, whereas DENV-2 and DENV-3 have been reported as the most prevalent DENV serotypes and are commonly associated with more severe disease [11-14]. Narvaez et al. [15] reported that DENV-2 and DENV-4 cause severe dengue fever as secondary infections, whereas DENV-1 and DENV-3 cause more severe disease as primary infections. DENV-5 has been reported to be responsible for milder illness [2].
DENV is a positive-sense single-stranded encapsulated RNA virus comprising three structural proteins (capsid, membrane, and envelope) and seven nonstructural proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5) [16]. Dengue fever is widely diagnosed using nonstructural protein 1 (NS1), a highly conserved glycoprotein among flaviviruses that is thought to be involved in viral RNA replication [17,18]. Clinical studies have shown that the dengue NS1 antigen is detectable early during the acute phase, up to 9 days after symptom onset, in primary and secondary DENV infections [19-22]. Serological tests using either enzyme-linked immunosorbent assays or immune chromatography-based rapid tests are commonly used to detect anti-DENV antibodies [10,23]. Molecular diagnostic tests, such as real-time reverse transcriptionpolymerase chain reaction (RT-PCR) are regarded as the gold standard for detecting DENV during the acute phase of the disease [24]. PCR-based techniques offer better sensitivity and more rapid turnaround times than viral isolation methods [24]. However, RT-PCR requires expensive equipment, reagents, and well-trained personnel [25], which is impractical in resource-limited settings.
Rapid diagnostic tests for detecting the DENV NS1 antigen have become commercially available and are widely used in countries with limited laboratory resources. Several studies have evaluated the clinical performance of NS1-antigen-based rapid diagnostic tests [26-30].
This study aimed to evaluate the clinical performance, including sensitivity and specificity, of the recently developed ImmuneMed Dengue NS1 Ag Rapid I test (ImmuneMed, Inc.) in Korea. Additionally, we evaluated the sensitivity of the tests for different DENV serotypes and when used at different periods from symptom onset to sample collection.
Go to : 
It is a diagnostic accuracy study, using retrospectively collected identified serum samples. Index test was a rapid diagnostic test and the reference test was a RT-PCR. It was described according to the STARD2015: An Updated List of Essential Items for Reporting Diagnostic Accuracy Studies available at https://www.equatornetwork.org/reporting-guidelines/stard/.
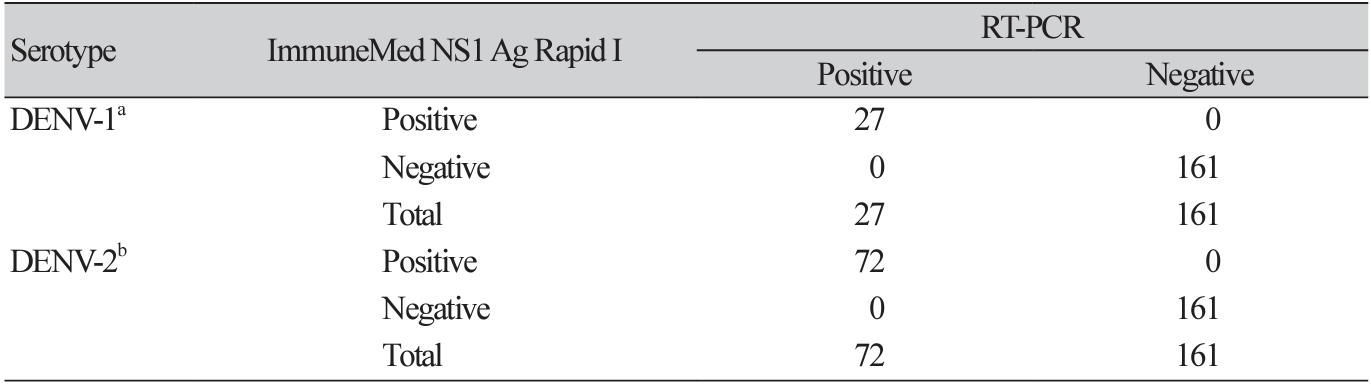
DENV-positive serum samples were transferred from institutes in foreign countries, including the Instituto de Investigación Nutricional, Peru; the National Institute of Hygiene and Epidemiology, Vietnam; and the University of Health Science, Laos. One hundred DENV-positive samples were obtained, of which 27 were confirmed to be DENV-1 and 72 were DENV-2. The serotype of one of the samples was unknown. In the control group, 161 DENV-negative samples were collected from Koreans who underwent health checkups and did not travel overseas lately.
Before the experiment, the positive and negative serum samples were randomized. The clinical performance was evaluated using these serum samples. The ImmuneMed Dengue NS1 Ag Rapid I test was performed according to manufacturer’s instructions. Briefly, two drops of serum were added to the device, and two drops of sample diluent were immediately added to the sample well. The results were interpreted visually after 20 min. If only the control band (‘C’) was stained, the test result was interpreted as negative, whereas if both the control and test bands (‘C’ and ‘T’) were stained, the test result was considered positive for DENV NS-1 antigen. Other staining patterns were considered invalid.
The AccuPower® ZIKV (DENV, CHIKV) multiplex real-time RT-PCR kit (Bioneer) was used to confirm the presence of DENV. Briefly, viral RNA was extracted from serum samples using ExiPrep™ 48 Dx (Bioneer), according to the manufacturer’s instructions. RT-PCR was performed using the Exicycler™ 96 (Bioneer) and ExiStation™ Manager software (Bioneer).
The results of the RT-PCR tests were defined as the gold standard. Standard diagnostic accuracy indices of sensitivity and specificity with corresponding 95% confidence intervals (CI) were calculated. We assumed that the serotypes of DENV and the period from symptom onset to sample collection might affect the performance of the ImmuneMed Dengue NS1 Ag Rapid I test. Therefore, we stratified the DENV positive samples according to serotypes (DENV-1 and DENV-2) and the period from symptom onset to sample collection (< 3 days and ≥ 3 days). We also calculated sensitivity and specificity in restricted subjects, including all negative and stratified positive subjects. All statistical analyses were performed using R software (R version 4.3.3).
Go to : 
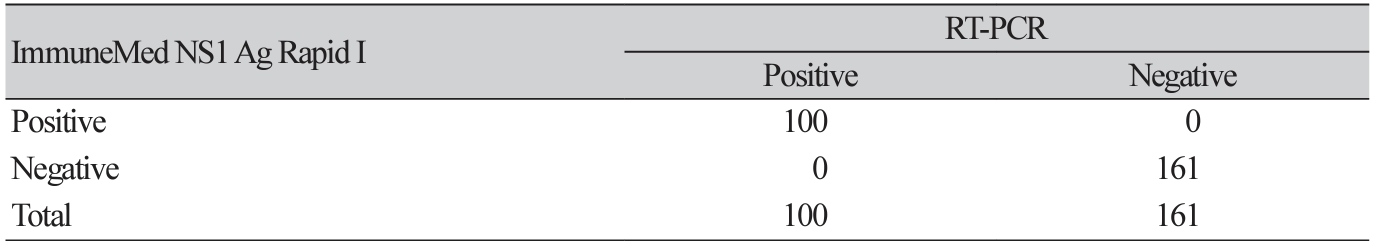
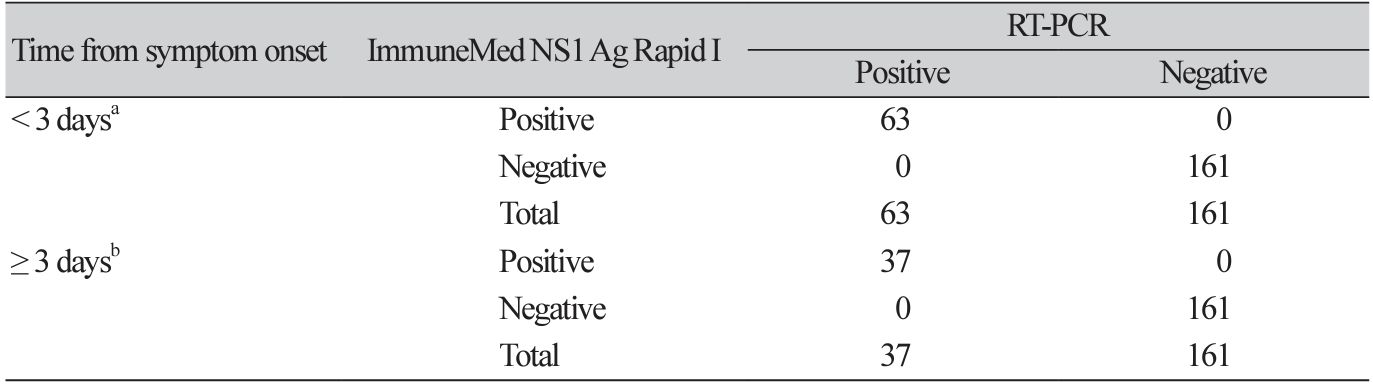
Overall, the results of the ImmuneMed Dengue NS1 Ag Rapid I and RT-PCR tests showed strong agreement. The sensitivity and specificity were 100% (95% CI, 96%-100%) and 100% (95% CI, 98%100%), respectively. Analyses of the clinical performance of the ImmuneMed Dengue NS1 Ag Rapid I and RT-PCR test are shown in Table 1. The samples were then grouped based on DENV serotype. The clinical performance of the ImmuneMed Dengue NS1 Ag Rapid I test for the DENV-1 and DENV-2 serotypes was also excellent (Table 2). We further evaluated the performance of the ImmuneMed Dengue NS1 Ag Rapid I test at different periods, from symptom onset to sample collection (< 3 days and ≥ 3 days). The sensitivity and specificity were 100% for each subgroup, as defined by clinical presentation at the time of sampling (Table 3).
Go to : 
Present study proves that the rapid diagnostic tests are useful for the detection of DENV, as they allow for the quick identification and isolation of suspected patients before RT-PCR results are available, especially in low- and mid-income countries or resource-limited settings.
Rapid diagnostic tests, which can be performed near a patient’s point-of-care, are widely adopted because of their practicality in preliminary diagnosis of dengue fever. Rapid assays that offer results within 15–30 min of sampling are highly desirable, particularly in cases where resources are limited or in remote settings. Several studies have evaluated the clinical performance of NS1 rapid diagnostic tests [31,32]. In this study, we evaluated the clinical performance of a commercial rapid diagnostic test, the ImmuneMed Dengue NS1 Ag Rapid I test, using sera collected from patients with confirmed dengue fever abroad and from healthy controls in Korea. Statistical analysis showed 100% sensitivity and 100% specificity, which is comparable to a previous study, in which the sensitivity and specificity were 97.4% (95% CI, 95.5%–99.5%) and 96.6% (95% CI, 93.2%–98.4%), respectively [30]. The agreement between the Immune-Med Dengue NS1 Ag Rapid I and RT-PCR test results was excellent. Both the ImmuneMed Dengue NS1 Ag Rapid I test and the RT-PCR test exhibited similar sensitivities (100%) for the detection of different serotypes of DENV and for serum samples collected either less than or more than 3 days after symptom onset. The strength of this study is that we performed the rapid NS1 Ag and RT-PCR tests simultaneously, which is practical for clinical practice.
This study has several limitations. First, we did not evaluate the sensitivity or specificity of the ImmuneMed Dengue NS1 Ag Rapid I test at detecting primary or secondary DENV infections. In addition, 72% (n = 72) of the samples were from DENV-2-infected patients. Owing to the limited number of DENV-1 samples and the absence of DENV-3 and DENV-4, the performance of the ImmuneMed Dengue NS1 Ag Rapid 1 test in detecting all DENV serotypes requires further investigation. Although we attempted to transfer the serum samples at a stable temperature in a frozen state, there might have been a temperature issue during storage or transportation. Nonetheless, the clinical performance of these serum samples was excellent. Furthermore, due to the limited volume of serum available, we were unable to compare with other rapid Ag tests.
Go to : 
Above results suggest that the sensitivity and specificity of the Immune-Med Dengue NS1 Ag Rapid test are comparable to those of the RT-PCR test. These results support the use of the ImmuneMed Dengue NS1 Ag Rapid test as a fast, inexpensive, and reliable point-of-care antigen-detection test for the diagnosis of dengue fever.
Go to : 
Ethics statement
This study was approved by the Institutional Review Board (IRB) of the Gyeongsang National University Changwon Hospital (IRB No. 2022-01-009).
Conflict of interest
The authors received financial supports from ImmuneMed Inc. for this study. Otherwise, no potential conflicts of interest relevant to this article were reported.
Acknowledgments
We would like to thank Soomin Kim, MS, at Gyeongsang National University Changwon Hospital for their dedication to performing the experiments, and Jieun Jang, Ph.D., at the Division of Clinical Research, Research Institute, National Cancer Center, Goyang, Korea, for performing the statistical analysis.
Funding
This study was supported by the Ministry of Trade, Industry, and Energy of Korea (grant number RS2024-00403563). The funders had no role in the study design, data collection and interpretation, or decision to submit the manuscript for publication.
Data availability
The datasets generated during the current study are available from the corresponding author upon request.
REFERENCES
1. Holmes EC. Molecular epidemiology and evolution of emerging infectious diseases. Br Med Bull 1998;54:533-43.
2. Mustafa M, Rasotgi V, Jain CS, Gupta V. Discovery of fifth serotype of dengue virus (DENV5): a new public health dilemma in dengue control. Med J Armed Forces India 2015;71:67-70.
3. Normile D. Surprising new dengue virus throws a spanner in disease control efforts. Science 2013;342:415.
4. Wahala WM and De Silva AM. The human antibody response to dengue virus infection. Viruses 2011;3:2374-95.
5. Guzmán MG, Kouri G, Valdes L, Bravo J, Alvarez M, Vazques S, et al. Epidemiologic studies on dengue in Santiago de Cuba, 1997. Am J Epidemiol 2000;152:793-9.
6. Winter PE, Nantapanich S, Nisalak A, Udomsakdi S, Dewey RW, Russell PK. Recurrence of epidemic dengue hemorrhagic fever in an insular setting. Am J Trop Med Hyg 1969;18:573-9.
7. Katzelnick LC, Gresh L, Halloran ME, Mercado JC, Kuan G, Gordon A, et al. Antibodydependent enhancement of severe dengue disease in humans. Science 2017;358:929-32.
8. Guzman MG and Harris E. Dengue. Lancet 2015;385:453-65.
9. Sangkawibha N, Rojanasuphot S, Ahandrik S, Viriyapongse S, Jatanasen S, Salitul V, et al. Risk factors in dengue shock syndrome: a prospective epidemiologic study in Rayong, Thailand: I. The 1980 outbreak. Am J Epidemiol 1984;120:653-69.
10. WHO. Dengue guidelines for diagnosis, treatment, prevention and control: new edition. Geneva: World Health Organization; 2009.
11. Balmaseda A, Hammond SN, Pérez L, Tellez Y, Saborío SI, Mercado JC, et al. Serotypespecific differences in clinical manifestations of dengue. Am J Trop Med Hyg 2006;74:449-56.
12. Vicente CR, Herbinger KH, Fröschl G, Romano CM, de Souza Areias Cabidelle A, Cerutti Jr. C. Serotype influences on dengue severity: a cross-sectional study on 485 confirmed dengue cases in Vitória, Brazil. BMC Infect Dis 2016;16:1-7.
13. Nisalak A, Endy TP, Nimmannitya S, Kalayanarooj S, Thisayakorn U, Scott RM, et al. Serotype-specific dengue virus circulation and dengue disease in Bangkok, Thailand from 1973 to 1999. Am J Trop Med Hyg 2003;68:191.
14. Vaughn DW, Green S, Kalayanarooj S, Innis BL, Nimmannitya S, Suntayakorn S, et al. Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. J Infect Dis 2000;181:2-9.
15. Narvaez F, Montenegro C, Juarez JG, Zambrana JV, Gonzalez K, Arguello S, et al. Dengue severity by serotype in 19 years of pediatric clinical studies in Nicaragua. medRxiv [Preprint]. 2024 [cited 2024 June 30]. Available from: https://www.medrxiv.org/ content/10.1101/2024.02.11.24302393v2.
16. Perera R and Kuhn RJ. Structural proteomics of dengue virus. Curr Opin Microbiol 2008;11:369-77.
17. Lindenbach BD and Rice CM. trans-Complementation of yellow fever virus NS1 reveals a role in early RNA replication. J Virol 1997;71:9608-17.
18. Mackenzie JM, Jones MK, Young PR. Immunolocalization of the dengue virus nonstructural glycoprotein NS1 suggests a role in viral RNA replication. Virology 1996;220:232-40.
19. Libraty DH, Young PR, Pickering D, Endy TP, Kalayanarooj S, Green S, et al. High circulating levels of the dengue virus nonstructural protein NS1 early in dengue illness correlate with the development of dengue hemorrhagic fever. J Infect Dis 2002;186:1165-8.
20. Duyen HT, Ngoc TV, Ha DT, Hang VT, Kieu NT, Young PR, et al. Kinetics of plasma viremia and soluble nonstructural protein 1 concentrations in dengue: differential effects according to serotype and immune status. J Infect Dis 2011;203:1292-300.
21. Young PR, Hilditch PA, Bletchly C, Halloran W. An antigen capture enzyme-linked immunosorbent assay reveals high levels of the dengue virus protein NS1 in the sera of infected patients. J Clin Microbiol 2000;38:1053-7.
22. Alcon S, Talarmin A, Debruyne M, Falconar A, Deubel V, Flamand M. Enzyme-linked immunosorbent assay specific to dengue virus type 1 nonstructural protein NS1 reveals circulation of the antigen in the blood during the acute phase of disease in patients experiencing primary or secondary infections. J Clin Microbiol 2002;40:376-81.
23. Pal S, Dauner AL, Mitra I, Forshey BM, Garcia P, Morrison AC, et al. Evaluation of dengue NS1 antigen rapid tests and ELISA kits using clinical samples. PLoS One 2014;9:e113411.
24. Lanciotti RS, Calisher CH, Gubler DJ, Chang GJ, Vorndam AV. Rapid detection and typing of dengue viruses from clinical samples by using reverse transcriptase-polymerase chain reaction. J Clin Microbiol 1992;30:545-51.
25. Wiwanitkit V. Dengue fever: diagnosis and treatment. Expert Rev Anti Infect Ther 2010;8:8415.
26. Yow KS, Aik J, Tan EYM, Ng LC, Lai YL. Rapid diagnostic tests for the detection of recent dengue infections: an evaluation of six kits on clinical specimens. PLoS One 2021;16:e0249602.
27. Blacksell SD, Jarman RG, Bailey MS, Tanganuchitcharnchai A, Jenjaroen K, Gibbons RV, et al. Evaluation of six commercial point-of-care tests for diagnosis of acute dengue infections: the need for combining NS1 antigen and IgM/IgG antibody detection to achieve acceptable levels of accuracy. Clin Vaccine Immunol 2011;18:2095-101.
28. Osorio L, Ramirez M, Bonelo A, Villar LA, Parra B. Comparison of the diagnostic accuracy of commercial NS1-based diagnostic tests for early dengue infection. Virol J 2010;7:361.
29. Liu LT, Chen CH, Tsai CY, Lin PC, Hsu MC, Huang BY, et al. Evaluation of rapid diagnostic tests to detect dengue virus infections in Taiwan. PLoS One 2020;15:e0239710.
30. Suputtamongkol Y, Avirutnan P, Wongsawat E, Kim YW. Performance of two commercial dengue NS1 rapid tests for the diagnosis of adult patients with dengue infection. Siriraj Med J 2020;72:74-8.
31. Mata VE, de Andrade CAF, Passos SRL, Hökerberg YHM, Fukuoka LVB, da Silva SA. Rapid immunochromatographic tests for the diagnosis of dengue: a systematic review and metaanalysis. Cad Saude Publica 2020;36:e00225618.
32. Lindenbach BD and Rice CM. trans-Complementation of yellow fever virus NS1 reveals a role in early RNA replication. J Virol 1997;71:9608-17.
Go to : 




 Citation
Citation Print
Print


 XML Download
XML Download