Abstract
Purpose
This study aimed to compare outcomes of opioid patients-controlled anesthesia (PCA) and intraoperative local anesthesia in terms of postoperative pain, lab results, patient surveys, and discharge scores to evaluate the feasibility of ambulatory laparoscopic cholecystectomy (LC).
Methods
Patients who underwent LC for acute cholecystitis were assigned to the outpatient surgery (OPS) group or inpatient surgery (IPS) group according to the surgeon. In the OPS group, a mixture of bupivacaine and epinephrine was injected into trocar sites and sprayed on the surgical dissection field. Oral opioid and analgesics were given twice a day. In the IPS group, patients received opioid PCA. Numeric rating scale (NRS) for walking, erythrocyte sedimentation rate (ESR), CRP, self-assessed survey on general physical condition and discharge, and discharge score of ambulatory surgery were assessed postoperatively.
Results
NRS was significantly lower in the OPS group. There were no significant differences in ESR and CRP between the groups. Self-assessed survey on general conditions and the possibility of discharge were significantly better in the OPS group. The discharge scores at 3, 6, and 9 hours were significantly higher in the OPS group.
Laparoscopic cholecystectomy (LC) is one of the most common surgeries in the elderly. Unlike the Western countries, in Korea, patients who undergo LC are typically hospitalized for several days because the hospitalization fee is not high. During hospitalization, patient-controlled anesthesia (PCA) using opioids is usually used. Consequently, most surgeons do not pay much attention to reducing postoperative pain during the surgery. In Western countries, LC is usually performed as an outpatient surgery (OPS), and various methods to reduce postoperative pain are employed [1]. At times, OPS for LC is required for patients but Korean surgeons are hesitant to explore an unfamiliar surgery system. In Korea, to date, no study has compared the surgical results after using opioid PCA and intraoperative local anesthesia used as per general protocol. If there are no differences in postoperative pain and the patient’s general condition, we can carry out ambulatory LC (ALC) with confidence. The purpose of this study was to evaluate the feasibility of ALC using bupivacaine instillation. We retrospectively compared the surgical outcomes in terms of postoperative pain, laboratory tests, patients’ surveys, and discharge scores for ambulatory surgery.
This was a retrospective study of patients who underwent LC for acute cholecystitis in the Chosun University Hospital from January 2022 to March 2022. The study protocol was approved from the Institutional Review Board of Chosun University Hospital (No. 2021-03-018). This study was performed in accordance with the Declaration of Helsinki and written informed consent was waived due to its retrospective nature. The inclusion criteria were patients who were discharged from the hospital within 5 days without complications, ≥18 years-old, normal postoperative mental status, American Society of Anesthesiologists physical status (ASA PS) classification of I, II, or III, and no previous anesthetic complications. One surgeon performed LC on the OPS base. The other 2 surgeons performed LC on an inpatient surgery (IPS) base. All surgeons carried out elective cholecystectomy as long as the operative risk was not so high.
All patients were premedicated with midazolam 0.04 mg/kg intramuscularly 30 minutes before the induction of anesthesia and were transferred to the operating room. Anesthesia was induced by propofol (1.0–2.0 mg/kg), and endotracheal intubation was conducted after adequate neuromuscular blockade by rocuronium bromide (0.6 mg/kg). Anesthesia was maintained with volatile anesthetics such as desflurane or sevoflurane with a 50% oxygen-air mixture and a target-controlled infusion of remifentanil to maintain vital signs within 30% of the baseline during surgery.
In the OPS group, the surgical procedure using 3 or 4 trocars was consistently performed by the same surgeon in all cases. Hasson technique was employed for establishing pneumoperitoneum, using a 10-mm umbilical trocar preceded by local anesthesia infiltration. The remaining 5-mm trocars were introduced under direct vision after the infiltration of local anesthesia in the ports. A 3–4 mL mixture of bupivacaine and epinephrine (1:100,000) was injected into whole layers of the abdominal wall of trocar sites including the peritoneum, muscle, subcutaneous layer, and skin. A 5 mL mixture of bupivacaine and epinephrine (1:100,000) was sprinkled on the surgical dissection field including the cystic plate of liver bed. Tramadol hydrochloride (50 mg) was given intravenously immediately after the operation. Oxycodone hydrochloride (10 mg) and acetaminophen (325 mg)/tramadol HCl (37.5 mg) were given orally twice a day immediately after the operation.
In the IPS group, patients received opioid analgesics using a PCA instrument (fentanyl bolus 50 µg as a loading dose; basal infusion 0.625 µg/kg/hr; intermittent bolus 1.0 µg/kg/hr; lockout time 10 minutes) for 2 days based on the hospital’s protocol.
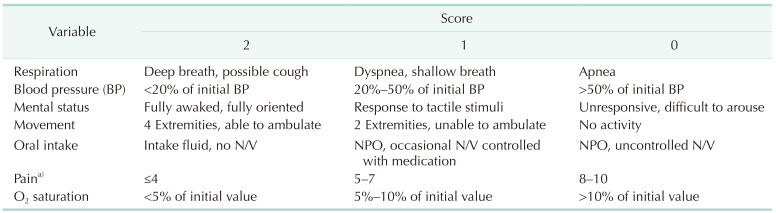
Pain scores evaluated by a numeric rating scale (NRS) for walking, erythrocyte sedimentation rate (ESR), CRP, and self-assessed surveys about general physical condition and the possibility of discharge were collected at 3, 6, and 9 hours postoperatively (Fig. 1). A scoring system for discharge of ambulatory surgery was assessed in both groups at 3, 6 and 9 hours postoperatively (Table 1). Statistical analyses were conducted using the t-test for NRS, ESR, CRP, and discharge score, as well as the Mann-Whitney U-test for the 5-point Likert scale in surveys. IBM SPSS Statistics ver. 24.0 (IBM Corp.) was employed for these analyses.
The mean age of the OPS vs. IPS was 75.45 years vs. 74.73 years (P = 0.874). Sex ratio of male:female was 15:15 vs. 13:17. Body mass index was 25.8 kg/m2
vs. 26.3 kg/m2 (P = 0.954). In the OPS group, 7 patients had hypertension and 3 patients had diabetes mellitus. In IPS, there were 6 patients with hypertension and 4 patients with diabetes mellitus. ASA PS grade of OPS vs. IPS was 2.3 vs. 2.1. Preoperative vital signs and the results of laboratory tests is shown in Table 2. The pain score calculated by NRS of OPS vs. IPS was 7.1 vs. 6.8. Twenty-eight patients in OPS and 26 patients of IPS had preoperative percutaneous transhepatic gallbladder drainage (PTGBD). Based on the revised American Association for the Surgery of Trauma (AAST) grading scale [23], 1 patient had grade II, 20 patients had grade III, 8 patients had grade IV, and 1 patient had grade V in the OPS group. In the IPS group, 2 patients had grade II, 18 patients had grade III, and 10 patients had grade IV. The grade of severity based on Tokyo Guideline 2018 [4] of IPS and OPS was 21 vs. 20 (grade I), 7 vs. 9 (grade II), and 2 vs. 1 (grade III). In the OPS group, 7 patients had a subtotal cholecystectomy and in the IPS group, 5 underwent subtotal cholecystectomy. The total operative time was 44.2 minutes vs. 48.8 minutes (P = 0.054) and the total amount of operative bleeding was 34.3 mL vs. 34.8 mL. Eleven patients in the OPS group and 12 patients in the IPS group had drainage after cholecystectomy. There was no statistical significance in preoperative characteristics (Table 2).
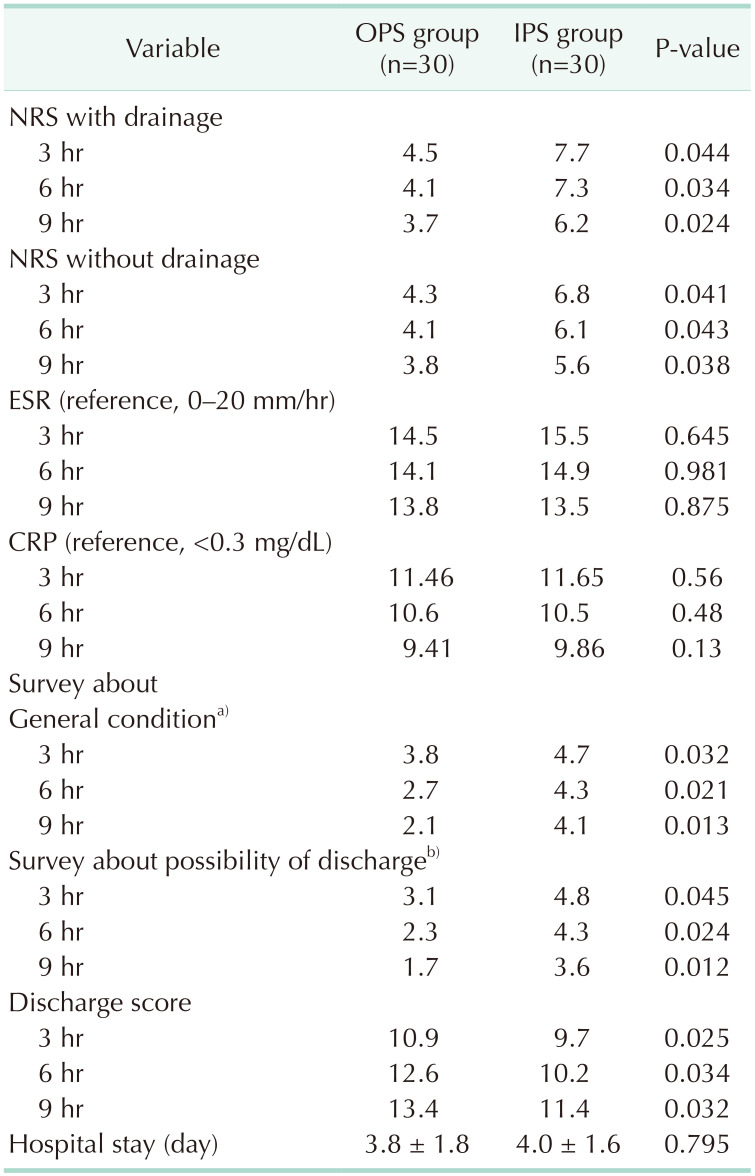
NRS with drainage at 3, 6, and 9 hours after operation in the OPS group were 4.5, 4.1, and 3.7. They were 7.7, 7.3, and 6.2 in the IPS group. NRS without drainage at 3, 6, and 9 hours in the OPS group were 4.3, 4.1, and 3.8. They were 6.2, 6.0, and 5.6 in the IPS group. Bupivacaine instillation was more effective when a drain was inserted. ESR at 3, 6, and 9 hours after operation in the OPS group were 14.5, 14.1, and 13.8 mm/hr; and in the IPS group, it was 15.5, 14.9, and 13.5 mm/hr. ESR at 3 hours was significantly lower in the OPS group. CRP at 3, 6, and 9 hours after operation in the OPS group were 11.46, 10.6, and 9.41 mg/dL; and in the IPS group, they were 11.65, 10.5, and 9.86 mg/dL. There was no significant difference. The survey scores about general condition at 3, 6, and 9 hours after operation in the OPS group were 3.8, 2.7, and 2.1 according to the 5-point Likert scale; and in the IPS group, they were 4.7, 4.3, and 4.1. The survey results about general conditions at 3, 6, and 9 hours were significantly better in the OPS group. The survey scores about the possibility of discharge at 3, 6, and 9 hours after operation in the OPS group were 3.1, 2.3, and 1.7 according to the 5-point Likert scale; and in the IPS group, they were 4.8, 4.3, and 3.6. The survey about the possibility of discharge at 3, 6, and 9 hours was significantly better in the OPS than IPS group. The discharge scores at 3, 6, and 9 hours after operation in the OPS group were 10.9, 12.6, and 13.4; and in the IPS group, they were 9.7, 10.2, and 11.4. The discharge scores at 3, 6, and 9 hours were significantly higher in the OPS than IPS group. The hospital stay of OPS vs. IPS was 3.8 vs. 4.0 days (Table 3).
An investigation on postoperative pain management for LC is not often done in Korea because of the routine employment of PCA after the operation. Hence, surgeons do not need to pay a lot of attention to pain management. This study which is majorly focused on intraperitoneal anesthetics instillation showed that intraoperative instillation of anesthetics reduced postoperative opioid consumption and was associated with less manifestation of pain postoperatively [5]. Contrary to Korea, pain management for LC is very popular in Western countries. The methods are also various; instillation of anesthetics in the port site, intraperitoneal nebulization of local anesthetics, erector spinae plane or transversus abdominis plane block, thoracoabdominal nerve block, and reduced intraabdominal pressure.
Intraoperative instillation of local anesthetics at the port site is the simplest method for postoperative pain management. Sharmin et al. [6] compared pain manifestation in patients with and without infiltration of bupivacaine at the port site after LC (groups I and II). Pain assessment using the NRS indicated a mean score of 2.55 at 6 hours for group I and 6.8 for group II. At 12 hours, the mean NRS score was 4.1 and 7.95. Group I exhibited a mean time of 13.85 hours for the first analgesic administration compared with a mean of 2.75 hours in group II. The mean duration between repeat doses of analgesic was 22 and 9.5 hours. Notably, 30.0% of patients in group I required a single dose of analgesic in the first 12 hours, whereas nearly 90.0% of patients in group II needed analgesics in the first 12 hours. Only 5.0% of group I patients needed analgesics within the first 6 hours, while all patients in group II required analgesics. Patients who were administered bupivacaine at port sites experienced lower postoperative pain and required fewer analgesic medications [6]. Several other studies have reported similar results indicating that intraperitoneal instillation reduced postoperative pain effectively [789].
Nebulizing local anesthetics in the peritoneal cavity is also effective in reducing postoperative pain. A double-blinded randomized controlled study showed significantly lower pain scores during rest and deep breathing up to 24 hours (P < 0.05). The pain score on movement was also lower, and the difference was statistically significant at 6 and 24 hours (P = 0.004 and P = 0.005, respectively). Tramadol consumption was lower, with a statistically significant difference at 24 hours (P = 0.044). That study showed that intraperitoneal nebulization of ropivacaine was both effective and safe in providing postoperative analgesia in LC [10]. Similar results have been reported in other studies [1112].
Facial plane blocks such as erector spinae, oblique transversus abdominis are other available methods. Mounika et al. [13] assessed the analgesic efficacy of ultrasound-guided erector spinae plane block (group E) and oblique subcostal transversus abdominis plane (OSTAP; group O) block in patients who underwent elective LC. That study evaluated the analgesic requirements and visual analogue scale (VAS) scores between the 2 groups and showed significantly lower VAS scores in group E on the first postoperative day. Group E maintained VAS scores <4 for the initial 24 hours, while group O had VAS scores ≥4 after 4 hours, leading to greater opioid needs. Tramadol was administered to 7 patients in group E compared to 62 patients in group O. The mean tramadol requirement for group E was 65.71 ± 26.3 mg, whereas group O required 114.56 ± 36.8 mg (P = 0.0012). Group O patients demanded tramadol more frequently than those in group E. Ultrasound-guided erector spinae plane block was found to provide superior pain control and reduce postoperative opioid consumption compared to OSTAP block in LC patients [13].
The effect of thoracoabdominal nerve block has been evaluated in many studies. In one study, participants were divided into 2 randomized groups: group M, comprising the modified thoracoabdominal nerve block through perichondrial approach group (n = 30), and the local infiltration (LI) group (n = 30). That study showed that the static NRS scores were significantly lower in group M during the first 4 hours postoperatively (P = 0.001). Additionally, there was a significant reduction in dynamic NRS scores in group M during the first 16 hours postoperatively (P = 0.001). The incidence of nausea was significantly higher in the LI group (12 patients vs. 5 patients, P = 0.047). Group M exhibited a significantly lower need for rescue analgesia (P = 0.009), and patient satisfaction scores were significantly higher in group M (P = 0.001) [14]. Lower abdominal pressure during LC is related to postoperative pain. In one study, 100 patients scheduled for elective LC were randomly assigned to either a low-pressure LC (LPLC) at 8 mmHg or a standard-pressure LC at 12 mmHg. Pressures were adjusted if vision was compromised. The primary outcomes of that study focused on postoperative pain and analgesia requirements at 4–6 hours and 24 hours. The results indicated a significant reduction in intraoperative visibility in LPLC (P < 0.01), leading to a higher incidence of pressure increases during operations (29% vs. 8%, P = 0.010). However, there were no significant differences in the duration of the operation or postoperative outcomes. Pain scores remained comparable at all time points across different pressure levels. Notably, the fentanyl requirement in the recovery room was more than 4 times lower in the 8-mmHg group than the 12-mmHg group (12.5 µg vs. 60 µg, P = 0.047). Despite similar pain scores, there was a significant reduction in fentanyl requirement and a lower incidence of nausea/vomiting in LPLC. Although LPLC compromised intraoperative visibility in some cases, there were no significant differences in complications, suggesting that LPLC is safe and potentially beneficial for all patients [15].
In Korea, there are few studies on intraoperative manipulation to reduce postoperative pain. Kwon et al. [16], in a study of 115 patients with ALC, reported that the success rate of OPS was 61.3%. Although no additional procedures for pain control were taken during the surgery, the pain scale and satisfaction rate were superior in ALC compared to admitted patients. In this study, NRS at 2, 4, and 10 hours were 3.3, 2.4, and 2.2. This is quite astonishing because, in many previous studies, the usual pain scale several hours after LC was about 6 to 9 without intraoperative manipulation of pain control, and with that pain level patients are usually not discharged for OPS [67]. NRS of the OPS group in this study was 4.5, 4.1, and 3.7 at 3, 6, and 9 hours postoperatively. It is quite difficult to find the reason for the difference because there was no detailed description of postoperative pain control and there were no standard discharge criteria in Kwon et al.’s study [16]. NRS is a quite subjective parameter and adjusting power to pain can be different according to the generation. Kang et al. [17], in a study of 40 patients, found that irrigation with 200 mL of saline containing 200 mg of lidocaine under the right hemidiaphragm and at the cholecystectomy site resulted in significantly lower abdominal pain scores compared to the control group during the initial 24 hours after surgery (P < 0.05). Pain scores of VAS were 5, 4.5, and 4 at 3, 6, and 9 hours postoperatively. Compared to this study, the pain score of our study was 4.5, 4.1, and 3.7 at 3, 6, and 9 hours postoperatively. This shows the instillation of local anesthetics in the trocar site will be helpful in reducing postoperative pain in LC [17]. This is the first study to evaluate the feasibility of ALC using combined intraoperative procedures to reduce postoperative pain in LC. Many previous studies reported that peritoneal irritation due to the dissection of surgical fields was related to postoperative pain. We used a combination method to reduce postoperative pain, i.e., the instillation of local anesthetics at port site and surgical dissection fields. This study is unique because we evaluated ALC with the standard discharge score system. Various ambulatory surgery systems are used in Korea. Most of the surgeons use a standard discharge scoring system. However, no previous study has used this discharge scoring system to evaluate the feasibility of ALC.
Preoperative PTGBD was nearly always performed. Most of the patients visited the emergency room (ER) for acute pain. We routinely carried out abdominal CT and if gall bladder is distended and NRS is higher than 6, we routinely inserted PTGBD and removed it just before the cholecystectomy. In my hospital, cholecystectomy is carried out electively. It takes about one week between ER admission and cholecystectomy. PTGBD seems to have some benefit in terms of pain and inflammation which is required to be validated.
The research results suggest that the administration of bupivacaine during surgery effectively controlled postoperative pain to the extent that patients could be discharged on the same day following cholecystectomy. Since the severity of preoperative cholecystitis and the inflammatory state of postoperation were similar between the 2 groups, and the surgery duration and intraoperative bleeding were comparable, it is believed that the bupivacaine administered during surgery had a significant impact. We believe that this study can be used for designing evidence-based protocols for using local anesthetics in OPS in LC.
This study has several limitations. It was a retrospective study and LC were carried out by 3 surgeons. The results need to be validated in a randomized controlled trial by a single surgeon. The quantity of local anesthetics was not as per a standard protocol and had to be adjusted according to the patient’s weight, age, and trocar size. The NRS is a subjective method to evaluate pain. Last, most LC surgeries are conducted on an inpatient basis, and the results are somewhat irrelevant if ambulatory surgery is not applied to LC.
In conclusion, intraoperative instillation of bupivacaine at the port site and dissection fields combined with postoperative oral opioids and NSAID has better effects on short-term postoperative pain than opioid PCA only. Pain scores, laboratory tests, patient surveys, and discharge criteria of ambulatory surgery all showed better outcomes with these simple intraoperative and postoperative procedures. Ambulatory LC appears to be a feasible procedure with the use of intraoperative instillation of bupivacaine and postoperative oral opioid and NSAID.
Notes
References
1. Jiménez Fuertes M, Costa Navarro D. Outpatient laparoscopic cholecystectomy and pain control: a series of 100 cases. Cir Esp. 2015; 93:181–186. PMID: 24629917.
2. Schuster KM, O’Connor R, Cripps M, Kuhlenschmidt K, Taveras L, Kaafarani HM, et al. Revision of the AAST grading scale for acute cholecyst it is with comparison to physiologic measures of severity. J Trauma Acute Care Surg. 2022; 92:664–674. PMID: 34936593.
3. Schuster KM, O’Connor R, Cripps M, Kuhlenschmidt K, Taveras L, Kaafarani HM, et al. Multicenter validation of the American Association for the Surgery of Trauma grading scale for acute cholecystitis. J Trauma Acute Care Surg. 2021; 90:87–96. PMID: 33332782.
4. Yokoe M, Hata J, Takada T, Strasberg SM, Asbun HJ, Wakabayashi G, et al. Tokyo Guidelines 2018: diagnostic criteria and severity grading of acute cholecystitis (with videos). J Hepatobiliary Pancreat Sci. 2018; 25:41–54. PMID: 29032636.
5. Kim TH, Kang H, Park JS, Chang IT, Park SG. Intraperitoneal ropivacaine instillation for postoperative pain relief after laparoscopic cholecystectomy. J Korean Surg Soc. 2010; 79:130–136.
6. Sharmin I, Mahmud F, Chowdhury FA, Khatun M, Alam MT, Chowdhury AK. Comparison of pain control and analgesic consumption with or without infiltration of bupivacaine at port sites after laparoscopic cholecystectomy. Mymensingh Med J. 2023; 32:1133–1139. PMID: 37777912.
7. Vijayaraghavalu S, Bharthi Sekar E. A comparative study on the postoperative analgesic effects of the intraperitoneal instillation of bupivacaine versus normal saline following laparoscopic cholecystectomy. Cureus. 2021; 13:e14151. PMID: 33927953.
8. Arabzadeh A, Seyedsadeghi M, Sadeghi N, Nejati K, Mohammadian Erdi A. Comparison of intraperitoneal bupivacaine and intravenous ketorolac for postoperative pain management following laparoscopic cholecystectomy. Anesth Pain Med. 2021; 11:e114623. PMID: 35291402.
9. Manan A, Khan AA, Ahmad I, Usman M, Jamil T, Sajid MA. Intraperitoneal bupivacaine as post-laparoscopic cholecystectomy analgesia. J Coll Physicians Surg Pak. 2020; 30:9–12.
10. Sandhya S, Puthenveettil N, Vinodan K. Intraperitoneal nebulization of ropivacaine for control of pain after laparoscopic cholecystectomy: a randomized control trial. J Anaesthesiol Clin Pharmacol. 2021; 37:443–448. PMID: 34759559.
11. Ingelmo PM, Bucciero M, Somaini M, Sahillioglu E, Garbagnati A, Charton A, et al. Intraperitoneal nebulization of ropivacaine for pain control after laparoscopic cholecystectomy: a double-blind, randomized, placebo-controlled trial. Br J Anaesth. 2013; 110:800–806. PMID: 23293276.
12. Szem JW, Hydo L, Barie PS. A double-blinded evaluation of intraperitoneal bupivacaine vs saline for the reduction of postoperative pain and nausea after laparoscopic cholecystectomy. Surg Endosc. 1996; 10:44–48. PMID: 8711605.
13. Mounika V, Sahu L, Mishra K, Mohapatra PS. A comparative evaluation of post-operative pain management using erector spinae plane block and oblique transverse abdominis plane block in patients undergoing laparoscopic cholecystectomy. Cureus. 2023; 15:e35750. PMID: 37020482.
14. Güngör H, Ciftci B, Alver S, Gölboyu BE, Ozdenkaya Y, Tulgar S. Modified thoracoabdominal nerve block through perichondrial approach (M-TAPA) vs local infiltration for pain management after laparoscopic cholecystectomy surgery: a randomized study. J Anesth. 2023; 37:254–260. PMID: 36575362.
15. Gin E, Lowen D, Tacey M, Hodgson R. Reduced laparoscopic intra-abdominal pressure during laparoscopic cholecystectomy and its effect on post-operative pain: a double-blinded randomised control trial. J Gastrointest Surg. 2021; 25:2806–2813. PMID: 33565010.
16. Kwon OH, Kim SK, Mun DB, Yun YK. Outpatient laparoscopic cholecystectomy at ambulatory surgical center. Korean J Hepatobiliary Pancreat Surg. 2001; 5:109–115.
17. Kang WJ, Kim SH, Lee SM. Effects of intraperitoneal lidocaine on abdominal and shoulder pain after a laparoscopic cholecystectomy. Korean J Anesthesiol. 2002; 42:198–204.
Table 2
Preoperative characteristics and surgical outcomes
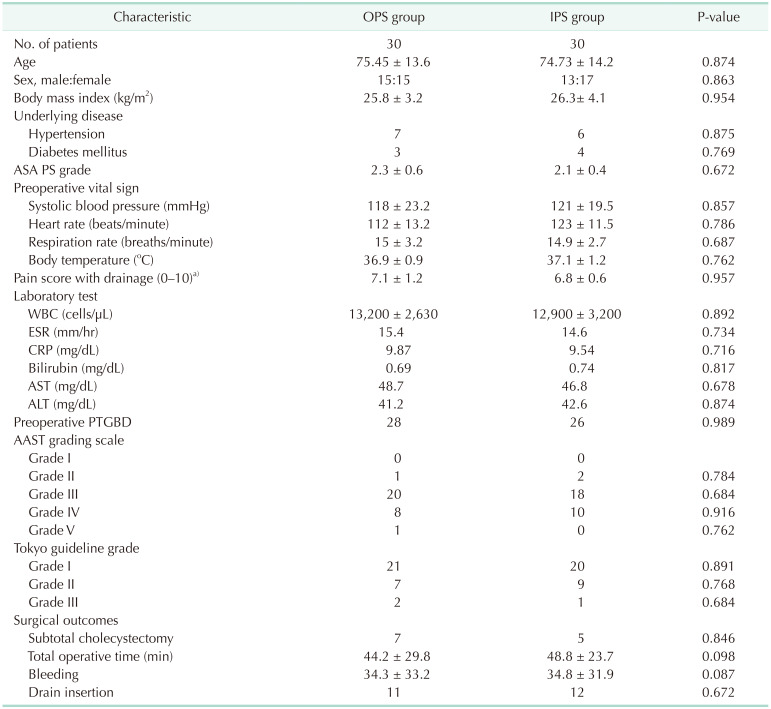
Values are presented as number only or mean ± standard deviation.
OPS, outpatient surgery; IPS, inpatient surgery; ASA PS, American Society of Anesthesiologists physical status; PTGBD, percutaneous transhepatic gallbladder drainage; AAST, The American Association for the Surgery of Trauma.
a)Visual analogue scale.




 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download