Introduction
The Korea Disease Control and Prevention Agency (KDCA) and the Ministry of Food and Drug Safety (MFDS) issued multiple press releases about safety concerns on a varicella vaccine product developed by a Korean manufacturer in June 2024, reassuring Korean people by explaining their safety assessment results.123 However, it is still unclear as to what kinds of safety concerns were detected as a signal for further evaluation since the KDCA and the MFDS said that the assessment was conducted due to the increase in adverse event reporting without mentioning a specific medical definition of the concern.1 The live attenuated varicella vaccine under the assessment, SKYVaricella™, was approved by the MFDS in 2018 and has been used for Korea National Immunization Program in children 12 months to 12 years of age in order to prevent varicella.4
The List of Potential Signals
A signal means information which suggests a new potentially causal association, or a new aspect of a known association between an intervention and an event or set of events.5 If there is a change in frequency, severity, or seriousness of a known association, it is considered a new aspect. This definition could be a primary tool to screen potential signals of the varicella vaccine.
A case of fatal disseminated varicella in a child vaccinated with SKYVaricella™ was reported from Korea in September 2023.6 The authors of the report suggested a potential causal relationship between this adverse event and problems with viral attenuation. Fatal varicella infection caused by the Oka strain is a known but rare complication, with only 6 cases documented in the United States since the first varicella vaccine was approved in 1995.78 If the above-mentioned case of fatal varicella infection in Korea implies a change in frequency or a deviation from the expected clinical course or outcomes, it may qualify as a signal.
Another candidate for a signal would be varicella-like rash, which is a known risk of varicella vaccines but was observed more frequently in the SKYVaricella™ group than in the control group in a phase 3 trial.9 In the SKYVaricella™ group, the rash was observed in 8 out of 251 subjects, whereas 2 out of 247 subjects reported the rash in the control group. Since the study was not powered to see the difference in safety profile, this numerical difference was not statistically significant. However, this may be a topic that mandates more data collection for continuous safety evaluation and risk minimization measures from a pharmacovigilance perspective.
The last candidate could be herpes zoster after vaccination. The KDCA and the MFDS said that 29 events of herpes zoster had been reported from the total varicella vaccine recipients since 2018, indicating a reporting rate is 0.0015% in total with 0.003% for the SKYVaricella™ recipients.1 In the business reports disclosed by the SKYVaricella™ manufacturer, the annual market shares of SKYVaricella™ between 2019 and 2023 range from 21% to 75% with the mean of 45.6%.10 When this market share data are applied to the herpes zoster reporting rate, it is estimated that herpes zoster could be 7.4 to 10.3 times more frequently reported in the SKYVaricella™ recipients. However, this hypothetical estimate should be interpreted cautiously, as it is based on the KDCA’s press release from spontaneous adverse event data, which are known to have several limitations.11 Nevertheless, a higher reporting rate would contradict the expected effect of viral attenuation, which is theoretically supposed to reduce the ability of vaccine viruses to reactivate.12
The Risk Management Plan (RMP) for SKYVaricella™
The safe and proper use of medicinal products is a common goal of all relevant stakeholders including patients, healthcare professionals, manufacturers and regulatory authorities, which is pursued through adhering to good pharmacovigilance practices regulated by laws and regulations locally and internationally. The MFDS, the Korean regulatory agency, adopted a risk management system following the ICH guidelines in 2015.13 The safety of SKYVaricella™ has been managed using this system by the manufacturer and the MFDS. If there is any significant update in safety characteristics, pharmacovigilance plans, or risk minimization measures, it is documented, conducted, and recorded in the frame of the system.
The RMP for SKYVaricella™ was established without any product-specific requirements for safety management at the time of the regulatory approval in 2018.14 Since then, there has been no update in the RMP, meaning no new safety concerns added, no new safety studies initiated, and no new risk minimization measures implemented. When comparing the SKYVaricella™ RMP with RMPs of other varicella vaccines, an obvious difference is noted in the safety specification section (Table 1).141516 In the SKYVaricella™ RMP, numerous non-important risks like pyrexia and myalgia are classified into important identified risks or important potential risks. In addition, the three potential safety concerns introduced above are not described, and risk minimization measures mentioned in the press releases of the KDCA and the MFDS are not included in the RMP.
Table 1
List of Safety Concerns of SKYVaricella™, Varivax®, and Varilrix
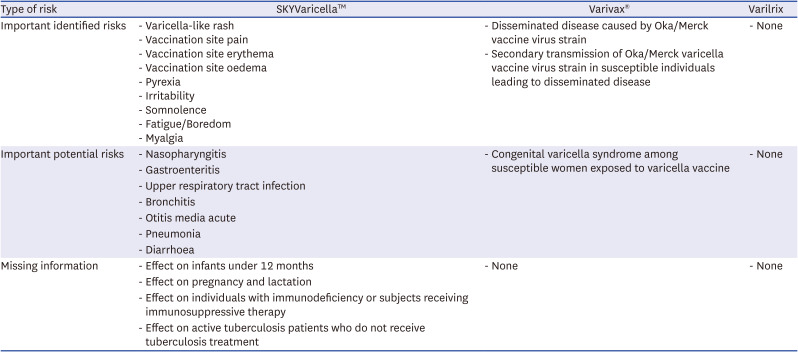
Accordingly, the existing RMP seems insufficient to assure the public and the press regarding vaccine safety management. Public trust in vaccine safety could be undermined by the misclassification of risks, inconsistencies between public concerns and KDCA assessments, and discrepancies between the RMP in effect and MFDS proposals.
The European Medicines Agency (EMA) Best Practice for Vaccine Safety Management
Preferred factors for a valid safety assessment and effective safety communication are exemplified in the safety assessment report for the AstraZeneca COVID-19 vaccine.17 The report presents assessment results from the respective safety databases of AstraZeneca and the EMA, allowing for a comparative analysis of both approaches. The report also clearly describes the medical definition of potential safety concerns, the statistics of relevant adverse events, and medical terms used to search for these events. The assessment utilized multiple data sources and valid scientific methods, such as the Bradford Hill criteria and the Medical Dictionary for Regulatory Activities.18 The proposed actions are logically and coherently structured to overcome the uncertainty caused by a lack of evidence, describing each stakeholder’s task in detail. All these aspects are transparently disclosed to the public, open to feedback, and documented in the RMP.
Proposals for the Varicella Vaccine Safety Management
The three potential safety concerns mentioned earlier, namely the fatal disseminated varicella infection, the increased varicella-like rash incidence, and the increased herpes zoster incidence, could be clinical manifestations originated from a common root cause, the unexpected high virulence of the vaccine virus. The currently available evidence seems to support the possibility of a virulence problem, and thus it is considered meeting the established definition of important potential risk.19
The study population for SKYVaricella™ is relatively small, making it challenging to sufficiently evaluate the vaccine’s safety profile.14 In addition, Korea’s vaccine adverse event management system is not optimized to detect potential safety signals sensitively.20 Therefore, cumulative safety data from all available sources are likely insufficient to assess the virus’s virulence characteristics from a clinical perspective. In this setting, various observational studies could be performed depending on the availability of diverse data sources. If the SKYVaricella™ vaccinated population can be identified from existing databases, a quick cohort study or a case-control study based on the secondary use of existing data would be a prioritized option. As a prospective study design, a varicella vaccine registry or a cohort study with primary data collection would be other feasible approaches to gather information on adverse events of special interest.
Safety communication using appropriate risk minimization measures is also an essential component. As a routine risk minimization measure, relevant safety information should be described in the warnings and precautions section of the prescribing information. If the regulatory agency judges that safety information should be delivered directly to healthcare professionals, direct healthcare professional communications like dear health care provider letters could be considered. To minimize the chance for the vaccine to be delivered to children with immunodeficiency or under the treatment of immunosuppressants, a system or program for controlled access could be implemented.
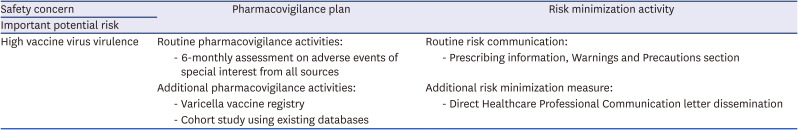
Finally, the RMP should serve as the cornerstone where all these proposed actions and plans are documented (Table 2), with its contents kept up-to-date and consistent with the prescribing information. Pharmacovigilance consists of scientific activities involving rigorous evaluation of the best available data and evidence generation for sound judgment. The last but not the least piece of the puzzle for good pharmacovigilance must be trust among all stakeholders, built on expertise, transparency, and a history of successfully managing safety issues.
Table 2
Summary of proposals to SKYVaricella™ risk management plan
Limitations
Thorough safety assessment requires accessibility to commercially confidential information and restricted data maintained in the regulatory database. This article is based solely on publicly available information, which the author acknowledges as an inherent limitation. Readers are urged to consider this constraint when interpreting the findings presented herein.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download