1. Alkahtani R, Stone S, German M, Waterhouse P. A review on dental whitening. J Dent. 2020; 100:103423. PMID:
32615235.
2. Naidu AS, Bennani V, Brunton JM, Brunton P. Over-the-counter tooth whitening agents: a review of literature. Braz Dent J. 2020; 31:221–235. PMID:
32667517.
3. Públio JDC, D’Arce MBF, Catelan A, Ambrosano GMB, Aguiar FHB, Lovadino JR, et al. Influence of enamel thickness on bleaching efficacy: an in-depth color analysis. Open Dent J. 2016; 10:438–445. PMID:
27708725.
4. Eriksen HM, Nordbø H. Extrinsic discoloration of teeth. J Clin Periodontol. 1978; 5:229–236. PMID:
363748.
5. Yildirim E, Vural UK, Cakir FY, Gurgan S. Effects of different over - the - counter whitening products on the microhardness, surface roughness, color and shear bond strength of enamel. Acta Stomatol Croat. 2022; 56:120–131. PMID:
35821723.
6. Omar F, Ab-Ghani Z, Rahman NA, Halim MS. Nonprescription bleaching versus home bleaching with professional prescriptions: which one is safer? a comprehensive review of color changes and their side effects on human enamel. Eur J Dent. 2019; 13:589–598. PMID:
31891975.
7. Aires CP, Sassaki GL, Santana-Filho AP, Spadaro ACC, Cury JA. Baccharis dracunculifolia-based mouthrinse alters the exopolysaccharide structure in cariogenic biofilms. Int J Biol Macromol. 2016; 84:301–307. PMID:
26691386.
8. Karadas M. Efficacy of whitening oral rinses and dentifrices on color stability of bleached teeth. Acta Biomater Odontol Scand. 2015; 1:29–34. PMID:
28642898.
9. Alqahtani MQ. Tooth-bleaching procedures and their controversial effects: a literature review. Saudi Dent J. 2014; 26:33–46. PMID:
25408594.
10. Gasparri F, Schemehorn BR, Zanardi A. Efficacy of teeth whitening with a mouthwash:
in vitro and
in vivo approaches. J Clin Dent. 2018; 29:13–17. PMID:
29758152.
11. Vieira-Junior WF, Ferraz LN, Giorgi M, Ambrosano G, Aguiar F, Lima D. Effect of mouth rinse treatments on bleached enamel properties, surface morphology, and tooth color. Oper Dent. 2019; 44:178–187. PMID:
29953341.
12. Carlin V, Matsumoto MA, Saraiva PP, Artioli A, Oshima CTF, Ribeiro DA. Cytogenetic damage induced by mouthrinses formulations in vivo and in vitro
. Clin Oral Investig. 2012; 16:813–820.
13. Lima FG, Rotta TA, Penso S, Meireles SS, Demarco FF.
In vitro evaluation of the whitening effect of mouth rinses containing hydrogen peroxide. Braz Oral Res. 2012; 26:269–274. PMID:
22641448.
14. Consolaro A. Mouthwashes with hydrogen peroxide are carcinogenic, but are freely indicated on the Internet: warn your patients! Dental Press J Orthod. 2013; 18:5–12. PMID:
24351145.
15. Ribeiro JS, de Oliveira da Rosa WL, da Silva AF, Piva E, Lund RG. Efficacy of natural, peroxide-free tooth-bleaching agents: a systematic review, meta-analysis, and technological prospecting. Phytother Res. 2020; 34:1060–1070. PMID:
31845403.
16. Chávez-González M, Rodríguez-Durán LV, Balagurusamy N, Prado-Barragán A, Rodríguez R, Contreras JC, et al. Biotechnological advances and challenges of tannase: an overview. Food Bioprocess Technol. 2012; 5:445–459.
17. Pauli MC, Kanemaru MYS, Vieira-Junior WF, Lima DANL, Bicas JL, Leonardi GR. Current status of whitening agents and enzymes in dentistry. Braz J Pharm Sci. 2022; 58:e19501.
18.
MC Pauli
JL Bicas
GR Leonardi
DANL Lima
RR Catharino
. UNICAMP. Process of obtaining dental extract, dental composition and its use in stain removal and tooth whitening. Brazil patent BR 102020022728-9. 2020. 11. 07.
19. Whitaker JR, Voragen AGJ, Wong DWS. Handbook of food enzymology. New York, NY: Marcel Dekker;2003.
20. Patil PA, Ankola AV, Hebbal MI, Patil AC. Comparison of effectiveness of abrasive and enzymatic action of whitening toothpastes in removal of extrinsic stains - a clinical trial. Int J Dent Hyg. 2015; 13:25–29. PMID:
25046241.
21.
M Wiliams
M Prencipe
JG Masters
. Colgate-Palmolive Company. Dual component dental composition containing enzyme. United States patent US 20040042977. 2004. 03. 04.
22. Gonçalves IMC, da Silva JA, Aguiar FHB, Lima DANL. Development of toothpaste formulations containing mineral clays as abrasive agents and their effects on the physical properties of dental enamel. J Esthet Restor Dent. 2024; 36:901–910. PMID:
38348937.
23. da Freiria ACB, Ortiz MIG, de Sobral DFS, Aguiar FHB, Lima DANL. Nano-hydroxyapatite-induced remineralization of artificial white spot lesions after bleaching treatment with 10% carbamide peroxide. J Esthet Restor Dent. 2022; 34:1290–1299. PMID:
36205242.
24. Ortiz MIG, Dos Santos JJ, Rodrigues-Filho UP, Aguiar FHB, Rischka K, Lima DANL. Maintenance of enamel properties after bleaching with high-concentrated hydrogen-peroxide gel containing calcium polyphosphate sub-microparticles. Clin Oral Investig. 2023; 27:5275–5285.
25. Govindarajan R, Revathi S, Rameshkumar N, Krishnan M, Kayalvizhi N. Microbial tannase: current perspectives and biotechnological advances. Biocatal Agric Biotechnol. 2016; 6:168–175.
26. Ortecho-Zuta U, de Oliveira Duque CC, Leite ML, Bordini E, Basso FG, Hebling J, et al. Effects of enzymatic activation of bleaching gels on hydrogen peroxide degradation rates, bleaching effectiveness, and cytotoxicity. Oper Dent. 2019; 44:414–423. PMID:
30444688.
27. Pérez MM, Pecho OE, Ghinea R, Pulgar R, Bona AD. Recents advances in color and whiteness evaluations in dentistry. Curr Dent. 2019; 1:23–29.
28. Torres CR, Perote LC, Gutierrez NC, Pucci CR, Borges AB. Efficacy of mouth rinses and toothpaste on tooth whitening. Oper Dent. 2013; 38:57–62. PMID:
22770430.
29. Oliveira J, Sarlo RS, Bresciani E, Caneppele T. Whitening efficacy of whitening mouth rinses used alone or in conjunction with carbamide peroxide home whitening. Oper Dent. 2017; 42:319–326. PMID:
28157418.
30. Karadas M, Duymus ZY.
In vitro evaluation of the efficacy of different over-the-counter products on tooth whitening. Braz Dent J. 2015; 26:373–377. PMID:
26312975.
31. Nahsan FPS, Reis MJO, Francisconi-Dos-Rios LF, Leão LV, Paranhos LR. Effectiveness of whitening mouthwashes on tooth color: an
in vitro study. Gen Dent. 2018; 66:e7–e10. PMID:
29513242.
32. Jaime IM, França FM, Basting RT, Turssi CP, Amaral FL. Efficacy of hydrogen-peroxide-based mouthwash in altering enamel color. Am J Dent. 2014; 27:47–50. PMID:
24902405.
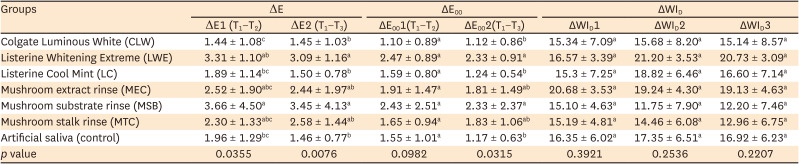
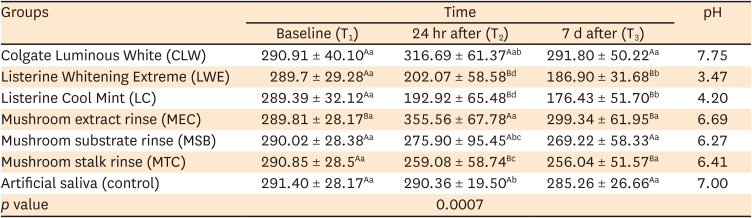




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download