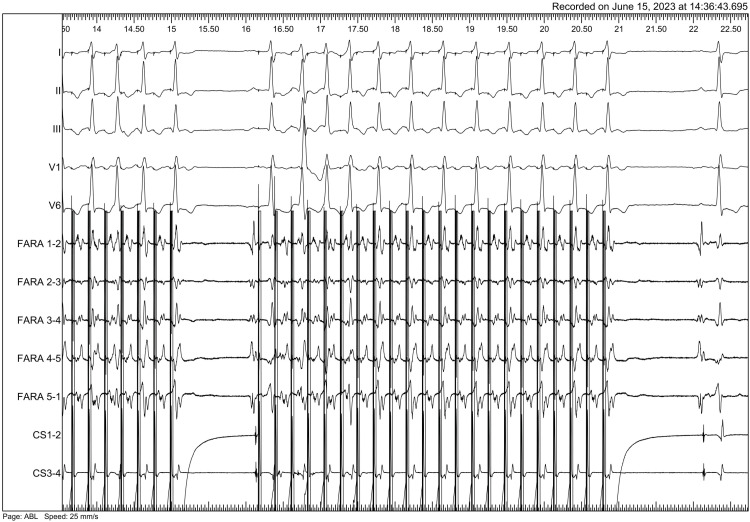
A 28-year-old man with congenital aortic stenosis, after biological prosthesis implantation and mitral annuloplasty (2011), aortic (SJM 23 mm) mechanical valve implantation and tricuspid annuloplasty (2015) and mitral (SJM 31 mm) mechanical valve implantation (MVR 2018) underwent pulsed field ablation (PFA) (Farawave; Boston Scientific, MA, USA) of atrial flutter. Previous extensive radiofrequency (RF) procedure failed to affect mitral isthmus (MI) dependent flutter cycle length. After cardioversion additional multiple RF applications from left atrium and coronary sinus were performed with no conduction block achieved. Four months later patient presented with poorly tolerated atrial flutter relapse and a history of 3 electrical cardioversions. After obtaining written consent PFA procedure was performed. Entrainment mapping from Farawave electrode placed at MI confirmed arrhythmia mechanism (Figure 1). Prior to PFA 2 mg of isosorbide dinitrate was administered intravenously to prevent coronary spasm.1) Seven applications were delivered at MI area. Electroporation resulted in prolongation of cycle length and arrhythmia termination (Figure 2). Aggressive stimulation protocol after ablation failed to induce any arrhythmia (Figure 3). The patient was evaluated after 6 and 10 months (Holter, echocardiography) and remains free from arrhythmia with no antiarrhythmic agents except beta-blockers. No dysfunction of the prosthetic valve was detected.
To the best of our knowledge this is the first report of peri-mitral atrial flutter electroporation in patient after mitral valve replacement.
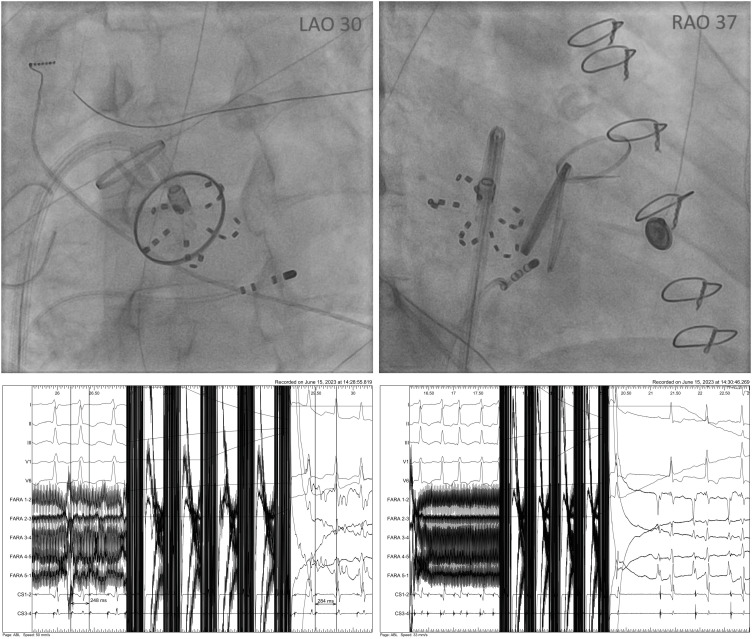
Mitral line RF ablation can be challenging especially in patients after MVR.2)3) In our case, the off-label PFA ablation proved to be effective and safe; however, this does not necessarily imply its superiority over other methods. Moreover the pentaspline catheter has to be positioned close to prosthetic valve but any contact has to be avoided (Figure 2, right anterior oblique).
Given the size of the electrode and the variability of patient anatomy, our results may not be replicable in every patient. Additional research is needed, particularly to address the long-term efficacy of PFA lesions.4)
Notes
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Data Sharing Statement: The data generated in this study is available from the corresponding author upon reasonable request.
Author Contributions:
Conceptualization: Urbanek P.
Data curation: Urbanek P, Bodalski R, Prokopowicz D.
Methodology: Urbanek P, Orczykowski M, Hasiec A, Jaworski K.
Supervision: Pręgowski J, Szumowski Ł.
Validation: Urbanek P.
Visualization: Urbanek P.
Writing - original draft: Urbanek P.
Writing - review & editing: Urbanek P.
References
1. Menè R, Boveda S, Della Rocca DG, et al. Efficacy of intravenous nitrates for the prevention of coronary artery spasm during pulsed field ablation of the mitral isthmus. Circ Arrhythm Electrophysiol. 2024; 17:e012426. PMID: 38095067.
2. Mountantonakis S, Frankel DS, Hutchinson MD, et al. Feasibility of catheter ablation of mitral annular flutter in patients with prior mitral valve surgery. Heart Rhythm. 2011; 8:809–814. PMID: 21236363.
3. Takigawa M, Vlachos K, Martin CA, et al. Acute and mid-term outcome of ethanol infusion of vein of Marshall for the treatment of perimitral flutter. Europace. 2020; 22:1252–1260. PMID: 32594180.
4. Gunawardene MA, Harloff T, Jularic M, et al. Contemporary catheter ablation of complex atrial tachycardias after prior atrial fibrillation ablation: pulsed field vs. radiofrequency current energy ablation guided by high-density mapping. Europace. 2024; 26:euae072. PMID: 38513110.
Figure 1
Entrainment mapping before first mitral isthmus application (post pacing interval and flutter cycle length).
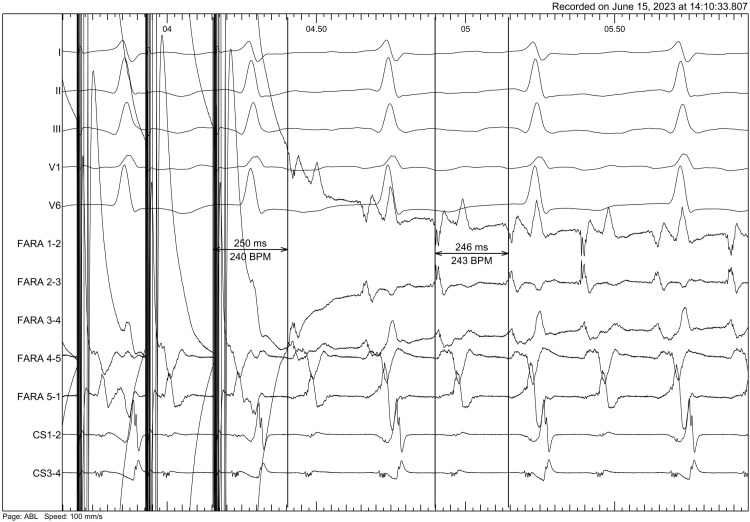
Figure 2
Farawave electrode positioned at the mitral isthmus (LAO 30º and RAO 37º view) proximal to prosthetic mitral valve in patient after mitral and aortic valve replacement and tricuspid valvuloplasty. Below: cycle length prolongation from 248 ms do 284 ms after 3rd application and arrhythmia termination after 7th mitral isthmus application.
LAO = left anterior oblique; RAO = right anterior oblique.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download