INTRODUCTION
Kawasaki disease (KD) is a systemic vasculitis that predominantly affects children under 5 years of age, as well as being the leading cause of acquired heart disease in developed countries.
1) KD is characterized by fever, rash, swollen lymph nodes, and inflammation of the blood vessels, particularly the coronary arteries.
1) Coronary artery aneurysms (CAAs) are among the most frequent complications of KD, affecting approximately 15–25% of untreated children and 3–5% of children treated with high-dose intravenous immunoglobulins (IVIGs).
2)3) CAAs are classified as small (internal diameter <5 mm), medium (internal diameter 5–8 mm), and giant (internal diameter >8 mm),
4) with the infiltration of inflammatory cells playing a crucial role in the pathogenesis of CAAs in patients with KD.
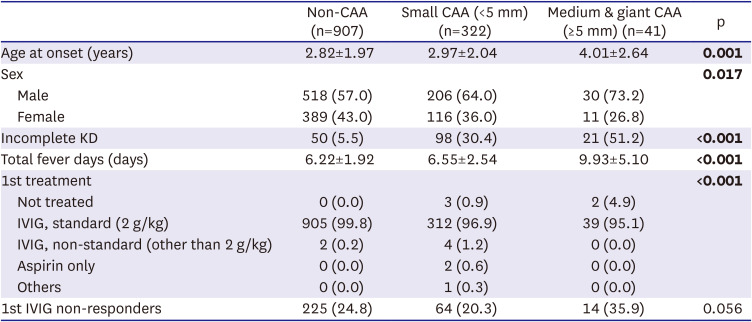
5)6) Several clinical factors, including male sex, resistance to IVIG treatment, prolonged duration of fever, and incomplete KD, have been found to influence the outcomes of CAAs in patients with KD.
7)8) Specifically, male patients with KD have shown a greater susceptibility to CAA formation than females with KD.
9)10) Although several genome-wide association studies (GWASs) using small sized samples have been performed to identify genetic loci associated with susceptibility to CAA formation in KD patients,
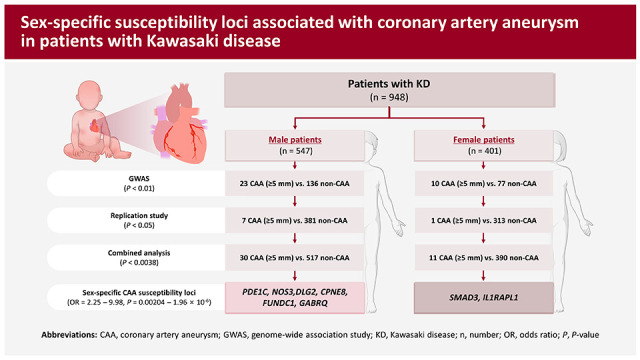
11)12)13)14) the genetic factors contributing to CAA formation in patients with KD remain incompletely understood. The present study was designed to identify additional susceptibility genes associated with CAA formation in KD patients by performing a sex-stratified GWAS using previous GWAS data,
15) comparing KD patients without CAA (controls) and with medium-sized aneurysms (diameter ≥5 mm) (cases). These findings revealed 6 male-specific and 2 female-specific CAA susceptibility genes in patients with KD, shedding light on the genetic factors involved in CAA formation in KD patients.
DISCUSSION
CAA is a serious complication of KD that can lead to significant morbidity and mortality, including increased risks of myocardial infarction, ischemic heart disease, sudden cardiac death, and the need for long-term monitoring and management.
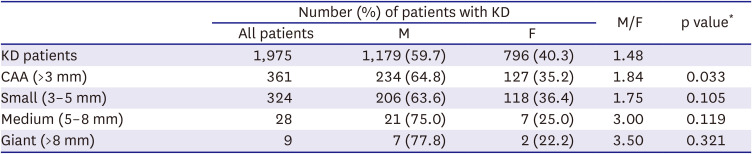
1)4)19) The incidence of CAA in KD patients varies based on sex, being much higher in males than in females.
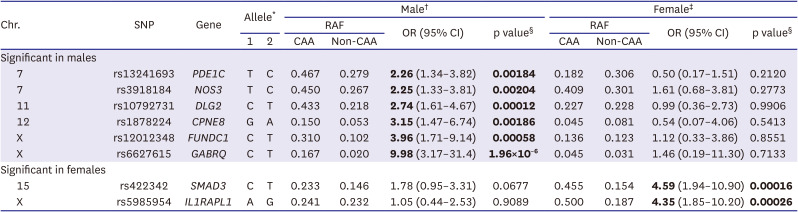
9)10) The sex-stratified GWAS performed in this study identified 6 male-specific (
PDE1C,
NOS3,
DLG2,
CPNE8,
FUNDC1, and
GABRQ) and 2 female-specific (
SMAD3 and
IL1RAPL1) CAA susceptibility loci.
Of these 8 CAA susceptibility genes, 3 (
SMAD3,
NOS3, and
PDE1C) were previously found to be associated with arterial aneurysms. Mutations in
SMAD3 have been linked to familial thoracic aortic aneurysms and dissections with intracranial and other arterial aneurysms.
20)21) Knockout of the
NOS3 gene in mice predisposed them to the formation of intracranial aneurysms.
22) The eNOS G894T polymorphism (rs1799983) has been identified as a mild predisposing factor for abdominal aortic aneurysms.
23) Additionally, the
PDE1C gene was shown to play a critical role in regulating vascular structure remodeling and function,
24) and to be expressed at high levels in human cardiac myocytes.
25)
PDE1C has also been implicated in the development of abdominal aortic aneurysms.
26) Interestingly, these 3 genes have also shown sex-specific differential expression and/or functions. For example,
PDE1C gene expression levels in arterioles were higher in adult male than in adult female rats,
27) with the T risk allele rs13241693 in the
PDE1C gene being associated with the higher expression of
PDE1C (
Supplementary Table 3). These findings suggest that higher
PDE1C gene expression and risk alleles for CAA in males may be involved in triggering CAA formation in patients with KD. Expression of the
eNOS gene was shown to be higher in endothelial cells of human females than males, accompanied by differences in activity and function in vitro and ex vivo.
28) In mouse models, differential sex-specific
Smad3 gene expression in the liver and sex-dependent functions of
Smad3 have also been observed.
29)30) By contrast, the T risk allele rs3918184 in the
NOS3 gene was found to be associated with higher expression of the neighboring
KCNH2 gene, located 12.7 kb upstream of
NOS3, in artery (aorta) tissue (
Supplementary Table 3). Furthermore, the T risk allele rs4725982 in the
KCNH2 gene was found to be significantly associated with CAA formation in patients with KD (OR, 2.77; p=0.0042;
Supplementary Table 2), although the level of significance did not reach the Bonferroni corrected significance threshold (p<0.0038 in the combined analysis). Therefore, the role of
NOS3 gene in CAA formation in patients with KD may be attributed to the direct effect of the
NOS3 gene itself and/or its synergy with the neighboring
KCNH2 gene that is involved in cardiovascular phenotypes, such as the QT interval, blood pressure, and electrocardiographic morphology (
Supplementary Table 4).
This sex-stratified genetic association study resulted in the identification of 3 candidate susceptibility loci (FUNDC1, GABRQ, and ILRAPL1) on the X chromosome that were associated with CAA in patients with KD. Variants on sex chromosomes are usually excluded from GWAS due to differences in gene dosage when compared with autosomal chromosomes. Because KD itself and CAA events in patients with KD were found to exhibit a male-dominant pattern, this study hypothesized that identifying CAA-associated variants and loci on sex chromosomes could provide valuable insights into the sex-specific occurrence of CAA in patients with KD. Nevertheless, the specific role of these 3 genes in CAA formation remains unknown. Similarly, although the present study found that the DLG2 and CPNE8 genes were associated with CAA formation in patients with KD and that the G risk allele of rs1878224 in the CPNE8 gene increased CPNE8 gene expression, the roles of these genes have not been determined. Additional studies are needed to evaluate the involvement of these genes in CAA.
The present study focused on identifying CAA-associated variants with a strong effect size, a selected subset of KD patients having CAA of significant size (≥5 mm; only 1.87% of patients) was included in the genetic association study. This approach resulted in larger effect sizes and more significant p values than assessments of KD patients with CAAs >3 mm (data not shown), likely due to the enrichment of CAA-associated variants within the group containing larger CAAs. However, this approach also resulted to lower statistical power due to the small sample size.
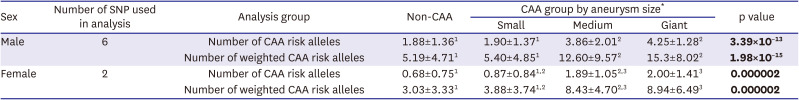
This sex-stratified association analysis resulted in the identification of 8 sex-specific susceptibility loci associated with the formation of CAAs in patients with KD. Interestingly, the sex-stratified association study, both for males and females, exhibited greater significance and larger effect sizes than the combined analysis of both sexes. Furthermore, the analysis of variants revealed sex-specific CAA susceptibility loci on the X chromosome. These findings indicate that sex-stratified association analysis and examination of variants located on the sex chromosomes are valuable methods for identifying susceptibility loci associated with sex-skewed phenotypes.
The present study had several limitations, primarily due to the small sample size resulting from the selection of KD patients with CAA, the division of these patients by sex, and the choice of a CAA size threshold having internal diameter ≥5 mm. Additionally, we also have potential for misclassification of CAAs, attributable to variations in the diagnostic processes during multi-center data collection. Furthermore, we did not use the Z-score system to classify the CAA. The Z-score system to classify the CAA in KD were used in 2017 for the first time by AHA and then subsequently the system was partly adapted by the Japanese Ministry of Health in 2020. The data collection form of our consortium (the Korean Kawasaki Disease Genetics Consortium) was made in 2008 and used it for 15 years to collect clinical samples. At that time the Z-score system was not introduced yet. That is the reason why we cannot use the Z-score system.
Although this approach allowed the identification of several sex-specific loci associated with susceptibility for CAA formation in patients with KD, the limited number of samples led to relatively lower statistical significance. Additional studies are required to validate these findings in independent sample sets or other cohorts of patients with KD.
In summary, this sex-stratified GWAS identified 6 male-specific (PDE1C, NOS3, DLG2, CPNE8, FUNDC1, and GABRQ) and 2 female-specific (SMAD3 and IL1RAPL1) CAA susceptibility loci in patients with KD, with all having large effect sizes. These findings highlight the effectiveness of sex-stratified association studies in identifying susceptibility genes associated with sex-skewed phenotypes, particularly in the context of male-dominant events associated with CAA formation in patients with KD.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download