INTRODUCTION
Type 2 diabetes mellitus (T2DM) places a high economic burden on healthcare systems due to diabetes-related, cardiovascular, and metabolic complications.
1) In 2017, approximately 462 million people worldwide were diagnosed with diabetes mellitus, corresponding to 6.28% of the overall population.
2) Diabetes is the leading cause of end-stage kidney disease and renal replacement therapy worldwide.
3) Chronic kidney disease (CKD) is also an important risk factor for cardiovascular disease, and individuals with both CKD and T2DM are at greater cardiovascular risk compared with T2DM alone.
4)
Sodium-glucose cotransporter-2 inhibitors (SGLT2i) have been shown to reduce cardiovascular mortality, major cardiovascular events (MACE), nonfatal myocardial infarction (MI), and nonfatal stroke in patients with T2DM.
5)6)7)8)9)10) In addition, some have shown favorable results of SGLT2i in patients with T2DM and CKD, with a reduction in the composite of cardiovascular mortality or hospitalizations for heart failure (HHF), MI, and stroke.
5)6)7)9)
However, the efficacy of SGLT2i in reducing other cardiovascular endpoints in this population is unclear. In the CREDENCE trial, for example, there was no significant difference in cardiovascular or all-cause mortality between patients with CKD and T2DM treated with SGLT2i vs. placebo.
5) Yet, such trials have studied patients with different cardiometabolic and renal function profiles. Given that the therapeutic effects of SGLT2i are influenced by glomerular filtration rate, there is still uncertainty regarding its actual impact in this population.
11)
Previous meta-analyses on the cardiovascular effects of SGLT2i in patients with T2DM and CKD have also shown conflicting results.
12)13) Those studies, however, were published before large prospective multicenter trials in this patient population, such as EMPA-KIDNEY, SCORED, and VERTIS CV.
7)9)10)
Therefore, we aimed to undertake a systematic review and updated meta-analysis assessing the clinical cardiovascular outcomes of SGLT2i compared with placebo in a population with T2DM and concomitant CKD.
METHODS
The prospective meta-analysis protocol has been uploaded to the International Prospective Register of Systematic Reviews (PROSPERO;
CRD42023401081).
Eligibility criteria
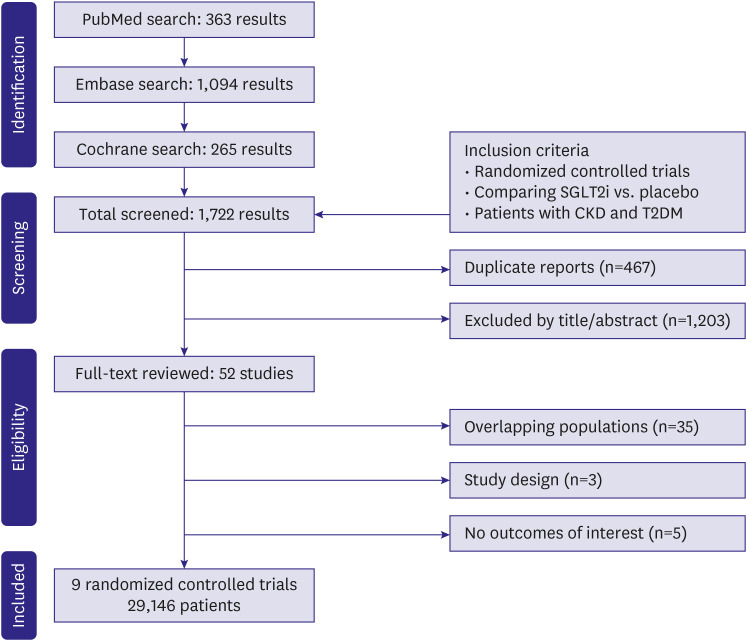
Inclusion in this systematic review and meta-analysis was restricted to studies that met all the following eligibility criteria: 1) randomized controlled trials (RCTs); 2) comparing SGLT2i with placebo; and 3) enrolling patients who had both CKD and T2DM. Definitions of CKD varied slightly among included studies, as outlined in
Table 1. Studies focused on a population of patients with CKD or T2DM were included only if outcomes were reported on a subgroup of patients with both CKD and T2DM. We excluded studies without outcomes of interest in this specific patient population.
Table 1
Individual study characteristics
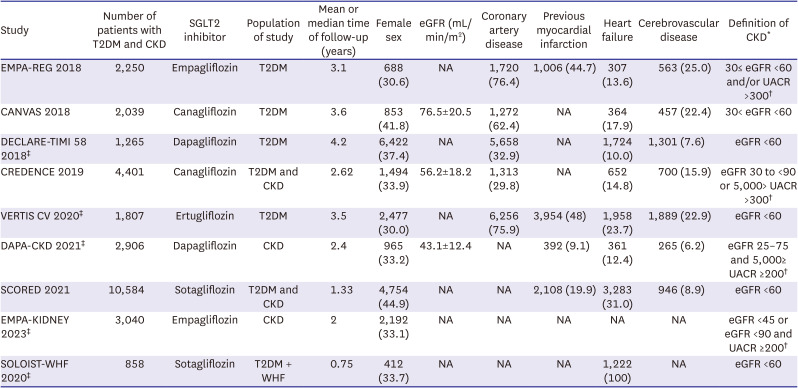
|
Study |
Number of patients with T2DM and CKD |
SGLT2 inhibitor |
Population of study |
Mean or median time of follow-up (years) |
Female sex |
eGFR (mL/min/m2) |
Coronary artery disease |
Previous myocardial infarction |
Heart failure |
Cerebrovascular disease |
Definition of CKD*
|
|
EMPA-REG 2018 |
2,250 |
Empagliflozin |
T2DM |
3.1 |
688 (30.6) |
NA |
1,720 (76.4) |
1,006 (44.7) |
307 (13.6) |
563 (25.0) |
30≤ eGFR <60 and/or UACR >300†
|
|
CANVAS 2018 |
2,039 |
Canagliflozin |
T2DM |
3.6 |
853 (41.8) |
76.5±20.5 |
1,272 (62.4) |
NA |
364 (17.9) |
457 (22.4) |
30< eGFR <60 |
|
DECLARE-TIMI 58 2018‡
|
1,265 |
Dapagliflozin |
T2DM |
4.2 |
6,422 (37.4) |
NA |
5,658 (32.9) |
NA |
1,724 (10.0) |
1,301 (7.6) |
eGFR <60 |
|
CREDENCE 2019 |
4,401 |
Canagliflozin |
T2DM and CKD |
2.62 |
1,494 (33.9) |
56.2±18.2 |
1,313 (29.8) |
NA |
652 (14.8) |
700 (15.9) |
eGFR 30 to <90 or 5,000> UACR >300†
|
|
VERTIS CV 2020‡
|
1,807 |
Ertugliflozin |
T2DM |
3.5 |
2,477 (30.0) |
NA |
6,256 (75.9) |
3,954 (48) |
1,958 (23.7) |
1,889 (22.9) |
eGFR <60 |
|
DAPA-CKD 2021‡
|
2,906 |
Dapagliflozin |
CKD |
2.4 |
965 (33.2) |
43.1±12.4 |
NA |
392 (9.1) |
361 (12.4) |
265 (6.2) |
eGFR 25–75 and 5,000≥ UACR ≥200†
|
|
SCORED 2021 |
10,584 |
Sotagliflozin |
T2DM and CKD |
1.33 |
4,754 (44.9) |
NA |
NA |
2,108 (19.9) |
3,283 (31.0) |
946 (8.9) |
eGFR <60 |
|
EMPA-KIDNEY 2023‡
|
3,040 |
Empagliflozin |
CKD |
2 |
2,192 (33.1) |
NA |
NA |
NA |
NA |
NA |
eGFR <45 or eGFR <90 and UACR ≥200†
|
|
SOLOIST-WHF 2020‡
|
858 |
Sotagliflozin |
T2DM + WHF |
0.75 |
412 (33.7) |
NA |
NA |
NA |
1,222 (100) |
NA |
eGFR <60 |
Endpoints and subanalyses
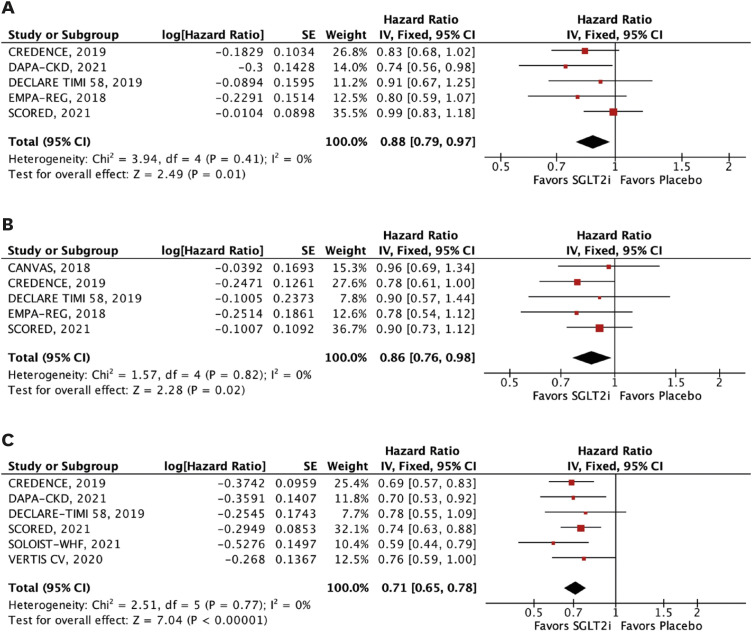
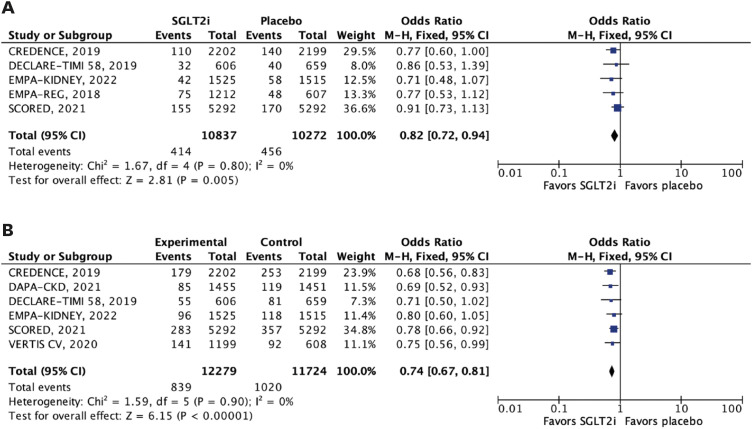
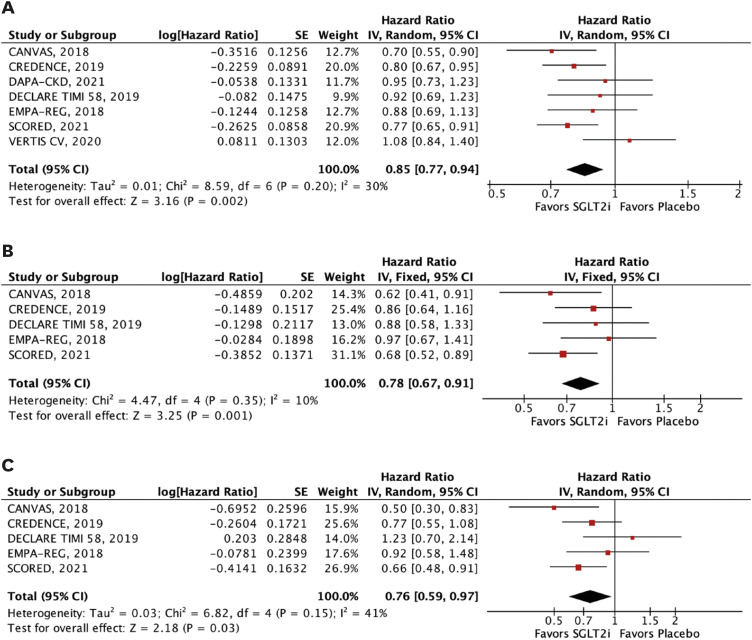
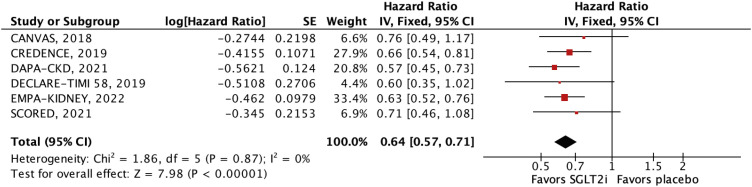
The efficacy outcomes were all-cause mortality, cardiovascular mortality, stroke, MI, major adverse cardiovascular events (MACE), HHF, and a composite of cardiovascular mortality or HHF or urgent visits for heart failure. MACE was defined as the 3-point composite of cardiovascular mortality, MI, or stroke. We performed subgroup analyses of the outcomes of interest according to estimated glomerular filtration rate (eGFR) <45 mL/min/1.73 m2 and 45 to 59 mL/min/1.73 m2.
To secondarily explore the renal effects of SGLT2i in these patients, we analyzed the composite of CKD progression. The definitions for MACE and CKD progression are displayed in
Supplementary Table 1.
Search strategy and data extraction
We systematically searched PubMed, Embase, and Cochrane Central Register of Controlled Trials from inception to February 2023 with the following search terms: 'RCT', ‘randomized’, ‘random’, ‘randomly’, ‘SGLT2 inhibitors’, ‘sodium-glucose cotransporter-2’, ‘SGLT2i’, ‘canagliflozin’, ‘dapagliflozin’, ‘empagliflozin’, ‘sotagliflozin’, ‘ertugliflozin’, ‘diabetes’, ‘type 2 diabetes’, ‘chronic kidney disease’, ‘CKD’, ‘nephropathy’, ‘cardiovascular’, ‘cardiac’, and ‘heart’. No filters or language limitations were applied in our search.
We also performed a technique of backward snowballing, searching for additional eligible studies through a review of the references from prior publications, including meta-analyses and included studies. Two authors (N.F. and M.M.G.) performed the literature search independently. Three authors (L.T., M.M.G., and N.F.) conducted the data extraction from included studies following predefined criteria and quality assessment. Eventual conflicts were resolved through consensus among the authors.
Risk of bias and sensitivity analyses
Quality assessment of RCTs was performed using the Cochrane Collaboration’s tool for assessing risk of bias in randomized trials (RoB-2), in which studies are scored as high, low, or unclear risk of bias in 5 domains: selection, performance, detection, attrition, and reporting biases. We also performed funnel plot analysis to appraise potential small study effects (publication bias) and sensitivity analyses using the leave-one-out strategy.
Statistical analysis
The systematic review and meta-analysis were performed in accordance with the Cochrane Collaboration and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement guidelines.
14) Endpoints were primarily analyzed with a hazard ratio (HR) with 95% confidence intervals (CIs) to compare treatment effects preserving time-to-event data. To include trials that did not report time-to-event analyses, we also computed the odds ratio (OR) for the outcomes reported in such trials. Cochran Q test and I
2 statistics were used to assess heterogeneity; p-values inferior to 0.10 and I
2≥25% were considered significant. We used a fixed-effect model for outcomes with low heterogeneity (I
2<25%). Otherwise, we chose the DerSimonian and Laird random-effect model. Review Manager 5.4 (Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark) and R (R Foundation for Statistical Computing, Vienna, Austria) version 4.1.2 were used for statistical analyses. Furthermore, we performed a weighted random-effects meta-regression to examine sources of heterogeneity and interactions in treatment effects for the outcome of MACE.
DISCUSSION
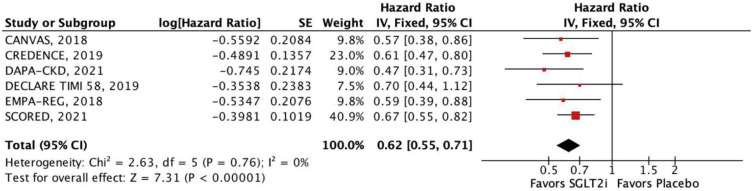
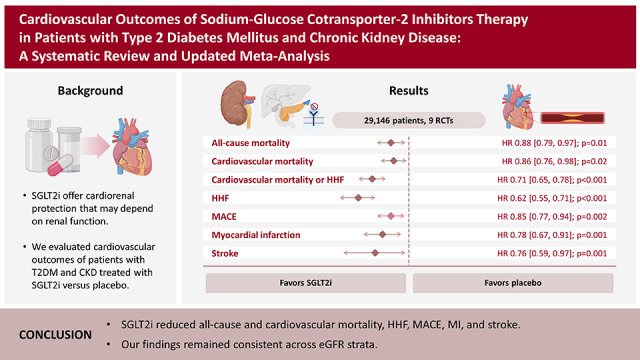
In this meta-analysis of nine RCTs, we compared SGLT2i with placebo in 29,146 patients with T2DM and concomitant CKD. The main findings were as follows: 1) SGLT2i were associated with a 12% relative reduction in all-cause mortality and 14% reduction in cardiovascular mortality; 2) HHF were also significantly reduced by SGLT2i, with a relative risk reduction of 38%; 3) SGLT2i were also associated with a significant reduction in MACE, stroke, and MI in this patient population; and 4) findings were generally consistent across subgroups of eGFR (<45 and 45–59 mL/min/1.73 m2). However, there was a significant interaction between strata in the outcome of stroke, with a benefit of SGLT2i in patients with lower eGFR (<45 mL/min/1.73 m2).
SGLT2i are widely used in the treatment of T2DM. They block glucose and sodium reuptake in the proximal renal tubule, thus promoting glycosuria.
15) A key factor for cardiovascular protection with SGLT2i use is the reduction of plasma volume due to its natriuretic and osmotic effects. This leads to a reduction in preload and afterload, which may be especially beneficial for decreasing the risk of HHF.
15) Other proposed mechanisms for its cardiovascular benefits include reduction of oxidative stress, renin-angiotensin-aldosterone system blockade, and attenuation of myocardial remodeling.
16)
As for renal protective pathways, SGLT2i improves renal oxygenation and reduces glomerular hypertension and hyperfiltration by tubuloglomerular feedback reduction and improvement in the albuminuria, which are essential mechanisms of diabetic nephropathy pathogenesis and cardiovascular risk predictors in nondiabetic and diabetic patients.
17)18)19) Even beyond the scope of diabetes, this class of medications has been vastly studied in the past years, and several large-scale clinical trials have also shown cardioprotective and renoprotective effects in patients with heart failure and CKD, respectively.
6)8)10)20)
Other meta-analyses have studied the population of patients with CKD and T2DM, with conflicting results among them.
12)13)21) A previous meta-analysis found no statistical effect of SGLT2i on stroke and cardiovascular mortality outcomes, diverging from our findings significantly.
13) In addition, another meta-analysis found no significant benefit of SGLT2i therapy in stroke in patients with T2DM,
22) which contrasts with our findings for the subgroup of patients with eGFR inferior to 45 mL/min/m
2. One plausible explanation for our findings is the stratification according to CKD status and residual renal function, comprising a more homogeneous population in terms of baseline cardiovascular risk. As a result, the pooled population had a higher risk of incident MACE and stroke and, therefore, would benefit the most from SGLT2i therapy.
On the other hand, another meta-analysis found a significant benefit of SGLT2i therapy in terms of stroke for patients with lower eGFR. Our overall analysis further strengthens such findings by comprising a significantly larger population with the inclusion of the SCORED trial.
9) As the authors previously stated, the underlying mechanisms for reducing stroke remain unclear. Even so, many mechanisms have been hypothesized. For instance, patients with T2DM have a higher incidence of risk factors for stroke, such as atrial fibrillation,
23) and there is evidence of the benefit of SGLT2i therapy in terms of atrial fibrillation in this high-risk population.
24)
Moreover, SGLT2i have been associated with reductions in endothelial dysfunction, inflammation, body weight, and blood pressure, which may play a role in the benefit of stroke rates in patients with lower eGFR and T2DM.
25) Although there are scarce data supporting this finding, SGLT2i may exert more pronounced stroke prevention effects in patients with T2DM and more severe renal impairment, potentially through their additional renal protective mechanisms.
26) Therefore, our results suggest a complex interplay between renal function, SGLT2i therapy, and stroke risk, highlighting the need for further investigation to elucidate the nuanced mechanisms involved.
Overall, several RCTs have been conducted since the publication of these previous meta-analyses, and the number of patients and outcomes investigated has significantly increased.
5)6)7)20) We also analyzed outcomes stratified according to renal function, showing consistent findings across different eGFR strata for the remaining outcomes.
Because SGLT2i inhibits glucose reabsorption in renal tubules, its action is expected to be eGFR-dependent.
15)19) SGLT2i also induces an acute, physiologic, and reversible reduction in eGFR, referred to as the eGFR dip. It was less clear, however, if this effect was of clinical significance until recent data—such as the EMPA-REG,
8) VERTIS-CV,
10) CREDENCE,
5) and most recently, EMPA-KIDNEY
7) studies—showed no association of SGLT2i use with progressive long-term kidney disease or worse cardiovascular outcomes. In fact, despite the initial transient reduction in eGFR, SGLT2i provides long-term renal benefits, such as attenuating CKD progression. Our meta-analysis demonstrates these effects and supports the efficacy of SGLT2i therapy even in the setting of lower baseline eGFR.
As recommended by the KDIGO 2022 guidelines on diabetes management in patients with CKD, SGLT2i therapy is a first-line therapy for reducing cardiorenal outcomes in this population, especially with regard to heart failure and the progression of CKD.
27) Our findings support this recommendation and add other key cardiovascular outcomes, such as cardiovascular mortality, stroke, and MI, to support SGLT2i use in this patient population.
Our study has limitations. First, the definition of CKD varied slightly among studies, including definitions related to eGFR or urine albumin-creatinine ratio, as shown in
Table 1. Second, the baseline cardiovascular risk may have also been heterogeneous in the patient population. DECLARE-TIMI 58,
6) EMPA-REG,
8) CANVAS,
28) and VERTIS CV
10) evaluated mainly patients with established cardiovascular disease or atherosclerotic cardiovascular disease, in contrast to CREDENCE
5) and DAPA-CKD,
29) which included patients with lower cardiovascular risks. Third, only a few studies reported data for the analyzed subgroups, which could lead to reduced statistical power in these analyses. Nevertheless, for the most part, the results remained statistically significant even in the subgroup analysis stratified by eGFR.
Finally, we performed a meta-regression for the outcome of MACE, which included only seven RCTs. The Cochrane Handbook recommends a minimum of ten studies for each examined covariate in meta-regression on the basis of the conventional rule of thumb of at least ten events per included covariate, which is used to reduce the likelihood of overfitting in logistic and Cox regression models. On the other hand, the handbook also emphasizes that it should not be taken as an inflexible rule. Based on a recent study that suggested that ordinary least squares linear regression may require considerably fewer observations per covariate than weighted random-effects meta-analysis, we decided to conduct meta-regressions using the results of seven large clinical trials.
30) However, whether this also applies to our weighted meta-regression remains uncertain. As a result, these findings should only be viewed as a sensitivity analysis.
In this meta-analysis of 29,146 patients with T2DM and CKD, SGLT2i reduced all-cause and cardiovascular mortality, HHF, MACE, MI, and stroke across strata of eGFR. These findings support the use of SGLT2i as the mainstay therapy for these patients.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download