INTRODUCTION
Reducing low-density lipoprotein (LDL) cholesterol is a cornerstone in the prevention of atherosclerotic cardiovascular disease (ASCVD), a leading cause of morbidity and mortality worldwide.
1)2) Moreover, the concept of "lower is better" and "earlier better" has gained widespread acceptance, with evidence supporting more aggressive LDL cholesterol lowering in individuals at higher risk.
3)4) In context, the US Preventive Services Task Force has advised that for the primary prevention of cardiovascular disease (CVD), healthcare providers consider starting lipid-lowering treatments for adults between 40 and 75 years old who present with one or more CVD risk factors and have a 10-year risk of a cardiovascular event of 10% or higher.
5)
Statin therapy is a cornerstone in reducing LDL cholesterol levels, resulting in enhanced clinical outcomes for individuals with or without ASCVD.
3)6) Although extensive evidence supporting its efficacy has established statin therapy as a fundamental component of ASCVD management,
7)8) statin intolerance, a condition that encompasses a variety of unfavorable signs and symptoms including myalgia, myopathy, and elevated liver enzymes,
9)10) can impede adherence to treatment and restrict the therapeutic advantages of these medications. Under these conditions, ezetimibe has emerged as a valuable component in the management of dyslipidemia, specifically for individuals with ASCVD or those who are intolerant to statins.
11) The co-administration of ezetimibe and statin therapy has demonstrated supplementary lipid-lowering advantages, frequently leading to a more pronounced reduction in LDL cholesterol levels compared to statin monotherapy.
12)13) The utilization of this particular combination therapy has produced enhanced clinical results among individuals with ASCVD.
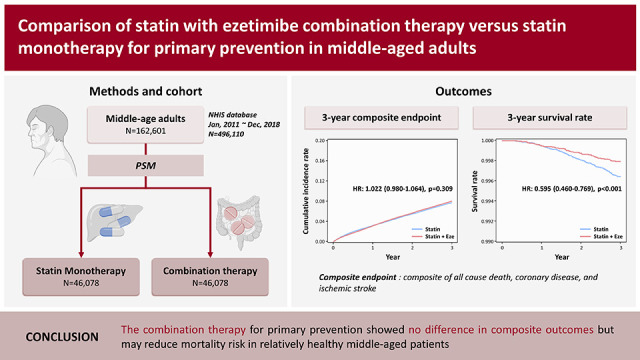
While combination therapy showed a good alternative option of reduction in LDL cholesterol levels than statin monotherapy in patients for secondary prevention, evidence regarding the effects of combination therapy on clinical outcomes in those for primary prevention strategy are limited. Hence, in the present study, we aimed to examine the impact of combination therapy as primary prevention strategy on clinical outcomes among a relatively healthy middle-aged population, utilizing data from the Korean National Health Insurance Service (NHIS).
METHODS
Ethical statement
The study protocol was reviewed and approved by the Institutional Review Board (IRB) of Kangbuk Samsung Hospital (KBSMC 2021-12-023). The IRB had granted a waiver of consent. All personal information of the participants was anonymized and de-identified.
Data source and selection of participants
In this retrospective cohort study, we utilized the Korean NHIS database, which contains not only records of healthcare utilization, such as diagnostic code, prescribed medication, and health examinations, but also death information and demographic data. The NHIS is the single national health insurer and obligatory healthcare insurance system,
14) covering nearly the entire Korean population. Thus, comprehensive and detailed studies can be conducted by aggregating health examination results and medication information.
15)
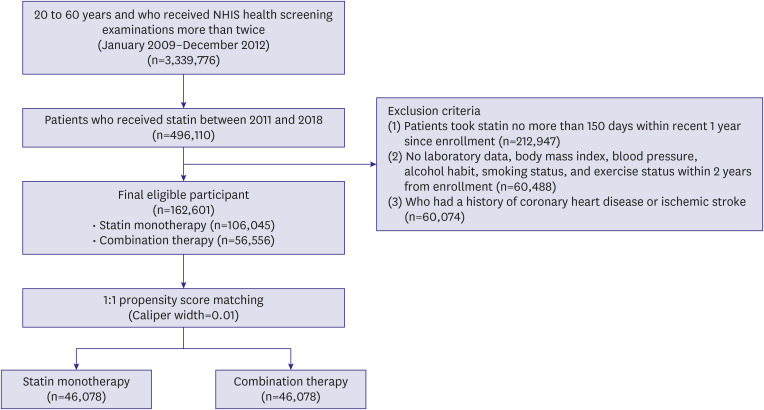
Data were collected from 3,339,776 patients aged 20–60 years who received NHIS health screening examinations more than twice between January 1, 2009 and December 31, 2012. Among these patients, we included those who had received statin between 2011 and 2018 (n=496,110). Patients were excluded if they 1) received statin for no more than 150 days within 1 year of enrollment (n=212,947); 2) had no laboratory data, including levels of LDL cholesterol, high-density lipoprotein (HDL) cholesterol, triglyceride, fasting blood glucose, creatinine; estimated glomerular filtration rate (e-GFR); body mass index (BMI); systolic blood pressure; diastolic blood pressure; waist circumference; alcohol intake; smoking status; and exercise within 2 years from enrollment (n=60,488); or 3) had a history of coronary artery disease or ischemic stroke (n=60,074). A total of 162,601 eligible patients were confirmed to be included in the study, who were categorized into the statin monotherapy (n=106,045) and combination therapy (n=56,556) groups (
Figure 1).
Figure 1
Flow chart of recruitment of the study participants.
NHIS = National Health Insurance Service.
Definitions
The operational definitions were divided into 3 parts, including demographics, medical history, and primary outcomes. Demographic factors included age (<40, 40–50, and ≥50 years), sex, and socioeconomic status (low, middle, and high) at enrollment. Laboratory data, including levels of LDL cholesterol (<130 and ≥130 mg/dL), HDL cholesterol (≤34 and >34 mg/dL), triglyceride (≤204 and >204 mg/dL), blood glucose, creatinine; e-GFR; BMI; systolic blood pressure; history of smoking, alcohol intake, and exercise, were recorded. The medical history was then identified based on claims of International Classification of Diseases 10th Revision (ICD-10) codes for each disease. Comorbidities were defined if patients had one or more billing records of hypertension (I10–I13, I15) or diabetes mellitus (E10–E14). Metabolic syndrome was defined as the presence of 3 or more of the following risk factors: waist circumference of ≥90 cm in men or ≥85 cm in women; triglyceride levels ≥150 mg/dL or intake of lipid-lowering medications; HDL cholesterol levels <40 mg/dL in men or <50 mg/dL in woman; systolic blood pressure ≥130 mmHg, diastolic blood pressure ≥85 mmHg, or intake of anti-hypertensive medications; and fasting blood glucose ≥100 mg/dL or intake of diabetes medications. The clinical outcomes of interest included 3-year all-cause death, incidental coronary heart disease (ICD-10 codes I20-I25 plus a coronary artery angiography procedure), and ischemic stroke (ICD-10 codes I63-I66 with brain imaging studies or procedures). Composite CVD events included any of the prespecified clinical events.
The primary and secondary outcomes of our study were 3-year composite outcomes and all-cause mortality, respectively. The occurrence of death is easily confirmed because the Korea NHIS subscribers lose their subscriber status immediately after death certification.
Statistical analysis
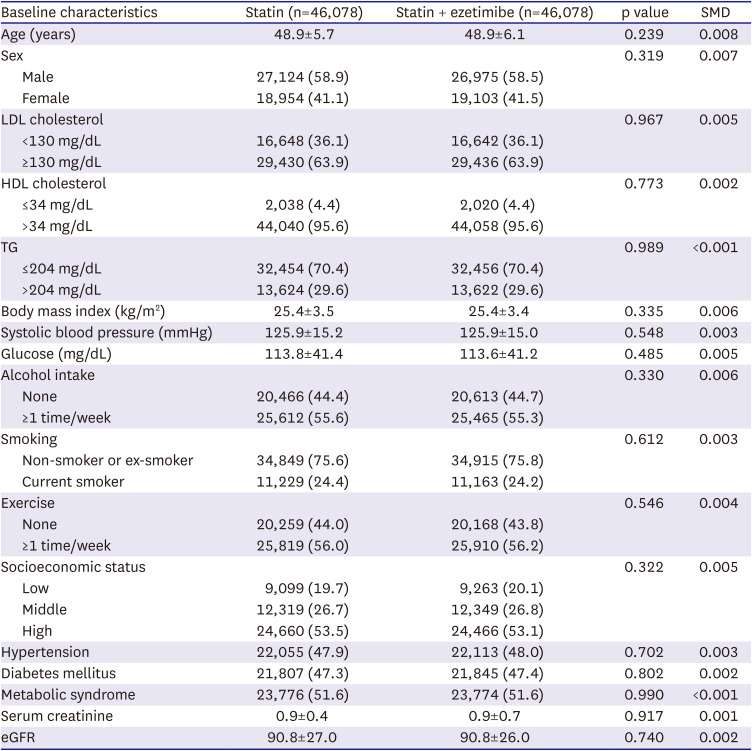
The Student’s t-tests and chi-square tests were utilized to compare baseline characteristics between the statin monotherapy and combination therapy groups. We performed a 1:1 propensity score matching for the patients using a caliper width of 0.01 to reduce selection bias. The propensity score was calculated using baseline characteristics, including sex, age, laboratory data (levels of LDL cholesterol, HDL cholesterol, triglyceride, fasting blood glucose, creatinine; e-GFR; BMI; systolic blood pressure), comorbidities (hypertension and diabetes mellitus), and presence of metabolic syndrome. Before and after propensity score matching histogram of 2 group with C-index was provided in
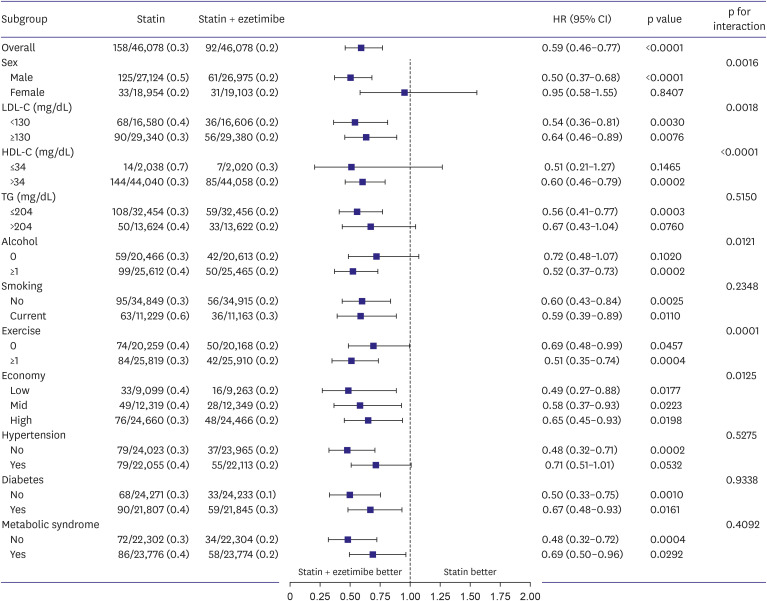
Supplementary Figure 1. Survival analyses were performed using the Kaplan–Meier method with a log-rank test to evaluate the difference in the cumulative incidence rates between the 2 groups and univariate Cox proportional hazard model with hazard ratio (HR) and 95% confidence interval (CI) was used to assess the risk of outcomes, including all-cause mortality, cardiac death, coronary heart disease, and ischemic stroke. Subgroup analyses were performed, which were stratified by potential effect modifiers, such as sex, levels of LDL cholesterol (<130 and ≥130 mg/dL), HDL cholesterol (≤34 and >34 mg/dL), triglyceride (≤204 and >204 mg/dL), alcohol intake status (0 and ≥1 a week), smoking status (non-smoker or former smoker and current), exercise status (0 and ≥1 a week), socioeconomic status (low, middle, and high), hypertension, diabetes mellitus, and metabolic syndrome. Two‐sided p value <0.05 were considered statistically significant for all comparisons. All statistical analyses were performed using SAS software (version 9.4; SAS Institute, Inc., Cary, NC, USA).
DISCUSSION
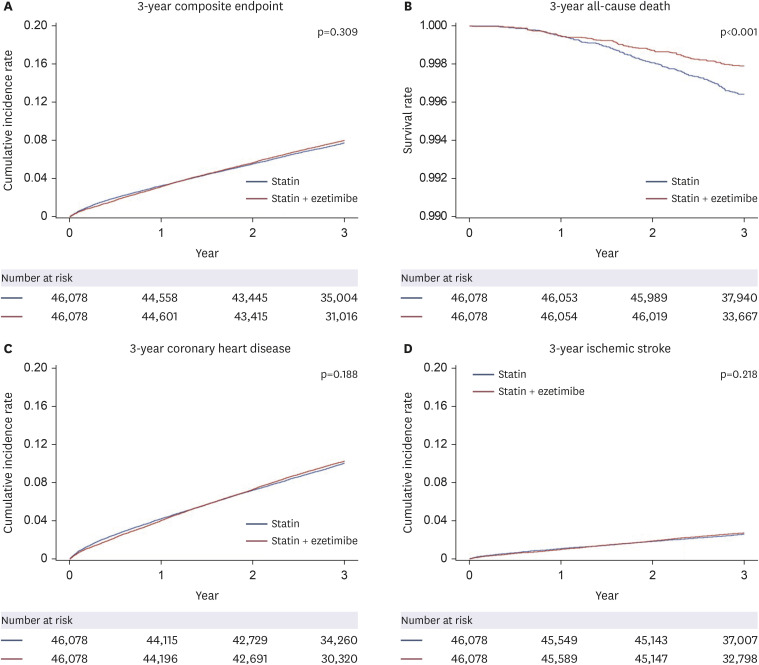
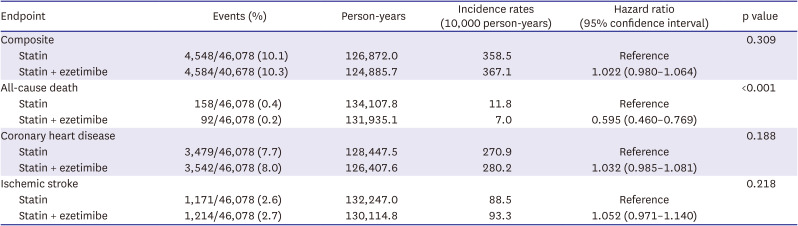
The present study examined the effects of statin monotherapy and combination therapy (statin with ezetimibe) on the clinical outcomes in a relatively healthy middle-aged population based on the Health Screening Cohort of the Korean NHIS. The study findings indicated that combination therapy as primary prevention strategy demonstrated no difference for composite outcomes compared to statin treatment alone. Meanwhile, compared to statin monotherapy, combining ezetimibe with statin therapy is significantly linked to a decreased risk of all-cause death. The results of the subgroup analyses indicated that the combination therapy group exhibited a lower incidence of all-cause death than the statin monotherapy group, which remained consistent across various subcategory variables.
According to the 2018 American College of Cardiology (ACC)/American Heart Association (AHA) and 2019 European Society of Cardiology (ESC)/European Atherosclerosis Society (EAS) dyslipidemia guidelines, the recommended treatment for patients with ASCVD is administration of moderate or high-intensity statins, or maximally tolerated statin therapy, based on their risk stratification.
7)16) Moreover, based on various randomized controlled trials, these guidelines emphasize the use of statins in both primary and secondary prevention to enhance cardiovascular outcomes.
7)8)16)17) A meta-analysis suggested a significant correlation between reduction in the rate of death or major coronary events over a span of 5 years and a decrease in LDL cholesterol levels, irrespective of the initial lipid profile or other presenting characteristics.
18) In addition, in a large-scale registry, statin therapy demonstrated benefit in all-cause mortality, irrespective of a patient’s risk or sex, which is consistent with the findings of the present study.
19) Consequently, statin therapy has emerged as a prevalent approach for managing dyslipidemia and mitigating death and cardiovascular risk. Nevertheless, the residual risk of CVD following statin use remains to be a relevant factor to be taken into consideration. Non-statin lipid-lowering agents, such as ezetimibe and proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors, have exhibited potential in mitigating this residual cardiovascular risk by decreasing LDL cholesterol levels.
13)20)21)
Ezetimibe is an inhibitor of cholesterol absorption. Specifically, it selectively inhibits the absorption of both dietary and biliary cholesterol in the small intestines.
22) This inhibition ultimately results in a decrease in plasma LDL cholesterol levels.
12) The effectiveness of ezetimibe in decreasing LDL cholesterol levels has been firmly established through multiple clinical trials, such as the IMProved Reduction of Outcomes: Vytorin Efficacy International Trial, as well as in meta-analytical studies.
13)23) However, its positive impact on clinical outcomes was primarily observed when it was used as a secondary preventive strategy and not as primary prevention. The Ezetimibe Lipid-Lowering Trial On Prevention of Atherosclerosis in 75 or Older was conducted to examine the impact of ezetimibe as a primary prevention strategy.
24) In this study, a total of 3,796 Japanese adults aged 75 years or older who had hypercholesterolemia with no prior history of coronary artery disease were randomly assigned to receive either ezetimibe or standard treatment. The study demonstrated that the integration of ezetimibe into the conventional treatment regimen resulted in a 34% reduction in the probability of atherosclerotic incidents.
24) However, evidence supporting the use of ezetimibe in younger patients is scarce. While statin-based lipid-lowering therapy has proven effective for patients aged 40 to 75 years, both with and without ASCVD, data on the effectiveness of ezetimibe for primary prevention in middle-aged populations remain limited.
5) In context, the present study included a middle-aged population who received lipid-lowering therapy as a primary prevention strategy, and we observed a beneficial association with all-cause mortality but no difference in cardiovascular risk.
One possible interpretation of a beneficial association with all-cause mortality is that, compared to the statin monotherapy group, the combination therapy group may have exhibited a greater reduction in LDL cholesterol levels, subsequently resulting in better sustained outcomes. The combined therapeutic approach resulted in an additional 18% decrease in LDL cholesterol levels.
12) The observed reduction in LDL cholesterol levels in the combination therapy is comparable to the incremental decrease achieved through a 3-stage of doubling the statin monotherapy. According to a recent study, patients using a combination of moderate-intensity statin and ezetimibe exhibit a higher rate of LDL cholesterol target achievement and a lower incidence of intolerance than those of the patients receiving high-intensity statins.
25) The efficacy of combination therapy in reducing LDL cholesterol levels for primary and secondary prevention may result in a decreased cumulative risk of LDL-associated cardiovascular events. Additionally, ezetimibe is known to have fewer adverse effects compared to statins. Poor adherence to lipid-lowering therapy has been linked to an increased risk of all-cause mortality in patients with ASCVD.
26) In this context, the combination therapy of rosuvastatin with ezetimibe has been observed to improve medication compliance rates compared to taking rosuvastatin alone, which could potentially benefit all-cause mortality rates.
However, the 2018 ACC/AHA cholesterol and 2019 ESC/EAS guidelines still do not provide a specific recommendation for the use of ezetimibe in primary prevention.
7)16) Instead, the main emphasis of the guidelines pertains to the use of statin therapy for primary prevention. The recommendations are based on an individual's age, cholesterol levels, presence of diabetes, and estimated ASCVD risk. The guidelines do recognize the significance of non-statin therapies, such as ezetimibe, in specific circumstances. The AHA guidelines suggest that for individuals at intermediate risk who would benefit from greater reduction in LDL cholesterol levels and cannot tolerate high-intensity statins, supplementing a moderate-intensity statin with a non-statin medication, such as ezetimibe or a PCSK9 inhibitor (class IIb), may be reasonable.
7)17) Meanwhile, the ESC guidelines advocate the combination of ezetimibe with statin therapy (class IB) in cases where the maximum tolerated dose of statin fails to achieve the desired LDL goals.
8)16) The present study proposes that the use of combination therapy is linked to improved clinical outcomes in a middle-aged population, irrespective of the risk profile.
This study had several limitations. First, because our sample consisted only of East Asian populations, caution should be exercised when extrapolating our findings to other racial and ethnic groups. Second, since our results were based on a retrospective review of the Korean NHIS database, coding errors may have occurred. In our multivariate model, several parameters including age, sex, medical history, medical data, and social habits were controlled and assessed by propensity score matching to correct for confounding factors. Third, although we divided the patients into 2 groups based on having a prescription for at least 5 months, we were unable to assess the effects of changing medications or discontinuation over such an extended period of time. Additionally, levels of LDL cholesterol during follow-up for each treatment were not collected due to the retrospective nature of the study. Fourth, in our study, the definition of middle-aged was established as patients aged 20–60 years. Due to the initial design of our research, we were unable to verify if the associations observed would be consistent in the broader age range of 40–75 years. Finally, due to the limitations of a retrospective analysis, the rate of statin intolerance, classification of statin or its intensity were not assessed in this investigation. Thus, our results should be interpreted with caution and further randomized clinical trials are needed to confirm these findings.




 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download