Abstract
Purpose
This study was performed to investigate influencing factors of preoperative muscle mass-to-fat ratio (MMFR) and its impact on overall survival and postoperative complications of colon cancer.
Methods
Patients who underwent colectomy for stage I–III colon cancer at the Second Affiliated Hospital of Soochow University between January 2016 and December 2022 were included. The skeletal muscle and fat area at the third lumbar vertebra were measured with preoperative CT measurement. MMFR was defined as the ratio of skeletal muscle area to total fat area, and low MMFR was defined as the 2 lowest tertiles (≤0.585). Univariate and multivariable analyses were conducted to assess the impact of MMFR on overall complications and survival outcomes. Kaplan-Meier survival curves and log-rank test were used to compare the overall survival between high MMFR and low MMFR groups.
Results
A total of 885 patients were analyzed. Female sex, older age, high body mass index, sarcopenia, and high cancer stage were more likely to result in low MMFR. Complications, including intestinal fistula, chylous fistula and organ space surgical site infection were significantly higher in the low MMFR group. Low MMFR was an independent factor associated with overall complications (odds ratio, 1.940; 95% confidence interval [CI], 1.252–3.007; P < 0.01) and long-term survival (hazard ratio, 2.222; 95% CI, 1.443–3.425; P < 0.01). Furthermore, patients with high MMFR had a higher survival rate than patients with low MMFR (P < 0.01).
Colon cancer, a common gastrointestinal malignancy worldwide, is characterized by a poor prognosis and a low survival rate. Various factors, including the patient’s overall health, tumor stage, and postoperative complications, influence the clinical outcomes of colon cancer. Surgery is the primary treatment approach for most patients, and the TNM staging system is commonly used to determine treatment and prognosis [1]. However, patients with the same TNM stage may experience different clinical outcomes [2]. Therefore, a new prognostic indicator is needed to personalize treatment strategies for patients with colon cancer.
Several large studies have demonstrated a strong correlation between body composition, specifically skeletal muscle and fat, and oncological outcomes [3]. CT-based analysis of muscle and adipose tissue regions at the third lumbar (L3) provides valuable insights into body fat and muscle mass, making it a powerful tool for evaluating cancer patients. Sarcopenia, which refers to the progressive loss of muscle strength, quantity, and function, is commonly observed in patients undergoing surgery for various cancers [4]. Sarcopenia reflects aggressive cancer biology, systemic inflammatory responses, and increased metabolic activity due to muscle depletion [5]. It has been shown to be a reliable predictor of postoperative complications and long-term prognosis in patients with colon cancer [6]. Additionally, obesity has a stronger association with cancer mortality compared to sarcopenia [7]. Obesity can contribute to immune cell dysfunction in the tumor immune microenvironment and lead to increased cancer risk, accelerated tumor growth, and higher mortality rates in patients with gastrointestinal cancer [8]. There are currently different results regarding the impact of visceral or subcutaneous fat on prognosis in patients with colon cancer. Recent research found that high subcutaneous fat area (SFA) was correlated with better disease-free survival in patients with colon cancer [9]. Another research found that the increased subcutaneous fat in males and visceral fat in females are associated with a higher risk of death in patients with stage I–III colon cancer [10]. The muscle mass-to-fat ratio (MMFR), as an important indicator, has been shown to be useful in predicting cardiac dysfunction and insulin resistance [11]. However, most studies focused on the individual effects of skeletal muscle or fat on colon cancer [12]. Few studies examined the combined influence of skeletal muscle and fat on predicting the prognosis of colon cancer.
Therefore, this study aimed to investigate the role of MMFR in predicting postoperative complications and long-term survival in colon cancer.
The study received approval from the Ethics Committee of the Second Affiliated Hospital of Soochow University (No. JD-HG-2023-0011) and written informed consent was obtained from all the patients.
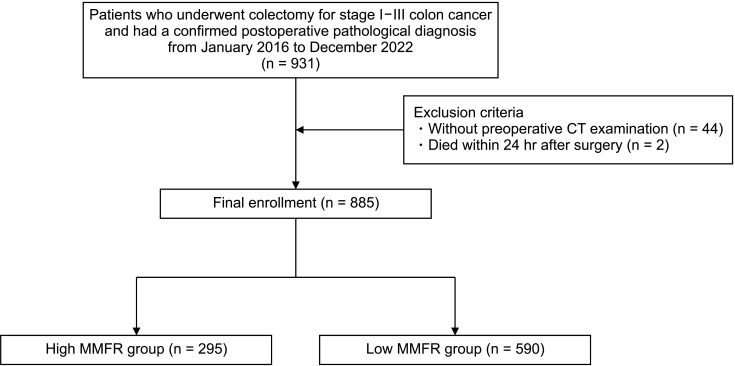
From January 2016 to December 2022, a total of 931 patients who underwent colectomy for stage I–III colon cancer at the Second Affiliated Hospital of Soochow University, and who had a confirmed postoperative pathological diagnosis, were included in the study. Exclusion criteria were patients without preoperative CT examination (44 patients) and patients who died within 24 hours after surgery (2 patients). Ultimately, a total of 885 patients were enrolled (Fig. 1).
Image acquisition digital imaging and communications in medical files for preoperative axial CT were obtained at mid-L3. As shown in Fig. 2, skeletal muscle area (SMA), visceral fat area (VFA), and SFA were quantified using SliceOmatic version 5.0 (TomoVision) according to the standard Hounsfield unit (HU) range. The tissue ranges of skeletal muscle, subcutaneous fat, and visceral fat are −29 to 150, −190 to −30, and −150 to −50 HU, respectively.
The patient characteristics and preoperative factors in this study included sex, age, body mass index (BMI), skeletal muscle index (SMI), presence of sarcopenia, MMFR, presence of preoperative serious underlying disease, tumor location, TNM stage, and preoperative laboratory indicators (measured 2 days before surgery). Intraoperative characteristics included operation methods (laparoscopy or open), operation time, amount of bleeding, blood transfusion status, vascular invasion, nerve invasion, and lymphatic invasion. Postoperative characteristics included hospital stay, postoperative hospital stay, intensive care unit (ICU) admission status, hospitalization expenses, postoperative laboratory indicators (measured 3–5 days after surgery), survival time, and overall complications. Overall complications included intestinal fistula, chylous fistula, incisional surgical site infection (SSI), organ space SSI, intestinal obstruction, gastroparesis, hemorrhage, thrombotic events, pneumonia, sepsis, reoperation, 30-day mortality, and other rare complications. SMI was defined as SMA (cm2)/height2 (m2). Sarcopenia was defined by sex-specific consensus [7]. Sarcopenia was defined as an SMI <41 cm2/m2 in female, <43 cm2/m2 in male with BMI <25 kg/m2, and <53 cm2/m2 in male with BMI >25 kg/m2. The total fat area (TFA) was calculated as the sum of VFA and SFA. The MMFR was defined as the ratio of SMA to TFA. High MMFR was defined as the highest tertile (>0.585), while low MMFR was defined as the 2 lowest tertiles (≤0.585).
The data were analyzed with IBM SPSS Statistics ver. 25.0 (IBM Corp.). The values were expressed as the median ± standard deviation for continuous variables or as a percentage of the group for categorical variables. Categorical variables were compared using the Fisher exact tests. Continuous variables that fit the normal distribution were compared using the Student t-test, and continuous variables that did not fit the normal distribution were compared using the Mann-Whitney U-test. Multivariate logistic regression was performed to examine the independent effect of risk factors on overall complications and the results were reported as the adjusted odds ratio (OR) with their corresponding 95% confidence interval (CI). Cox regression was performed to examine the independent effect of risk factors on survival time and the results were reported as the hazard ratio (HR) with their corresponding 95% CI. Only variables with a significance of 0.05 in the univariate analysis were included in the multivariate model. Survival curves were estimated with the Kaplan-Meier method and compared using the log-rank test. A P-value of <0.05 was considered to indicate statistical significance.
Out of the 885 patients enrolled, 295 patients met the criteria for high MMFR (>0.585). Among these patients, there were 226 males and 69 females. On the other hand, 590 patients were diagnosed with low MMFR, including 279 males and 311 females. As shown in Table 1, patients with low MMFR were significantly older than those with high MMFR (P < 0.01). Moreover, patients with low MMFR had a higher level of BMI (P < 0.01), preoperative lymphocyte count (P < 0.01), and globulin (P = 0.01), and had a higher incidence of sarcopenia (P < 0.01) than those with high MMFR. Additionally, the proportion of patients with low MMFR increased as the tumor stage advanced (P = 0.021). No significant differences were observed between patients with high and low MMFR in terms of other background and preoperative characteristics, such as tumor location, preoperative serious underlying disease, and other preoperative laboratory indicators.
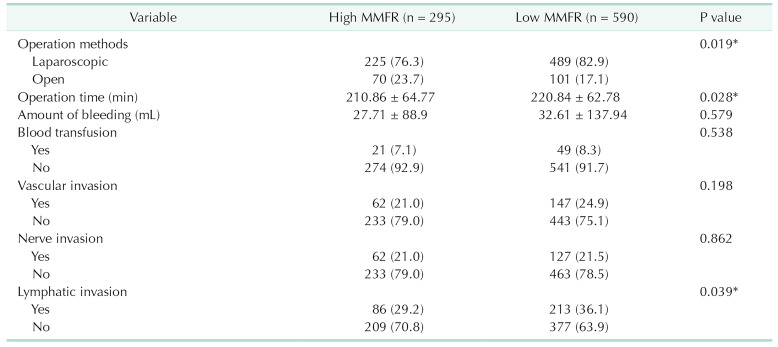
As shown in Table 2, open surgery and operation time were significantly higher in the low MMFR group compared to the high MMFR group (P = 0.019 and P = 0.028, respectively). The incidence of lymphatic invasion was also significantly higher in the low MMFR group (P = 0.039). However, no significant differences were found between patients with high and low MMFR in terms of other intraoperative features, such as blood loss, transfusion, vascular invasion, and nerve invasion.
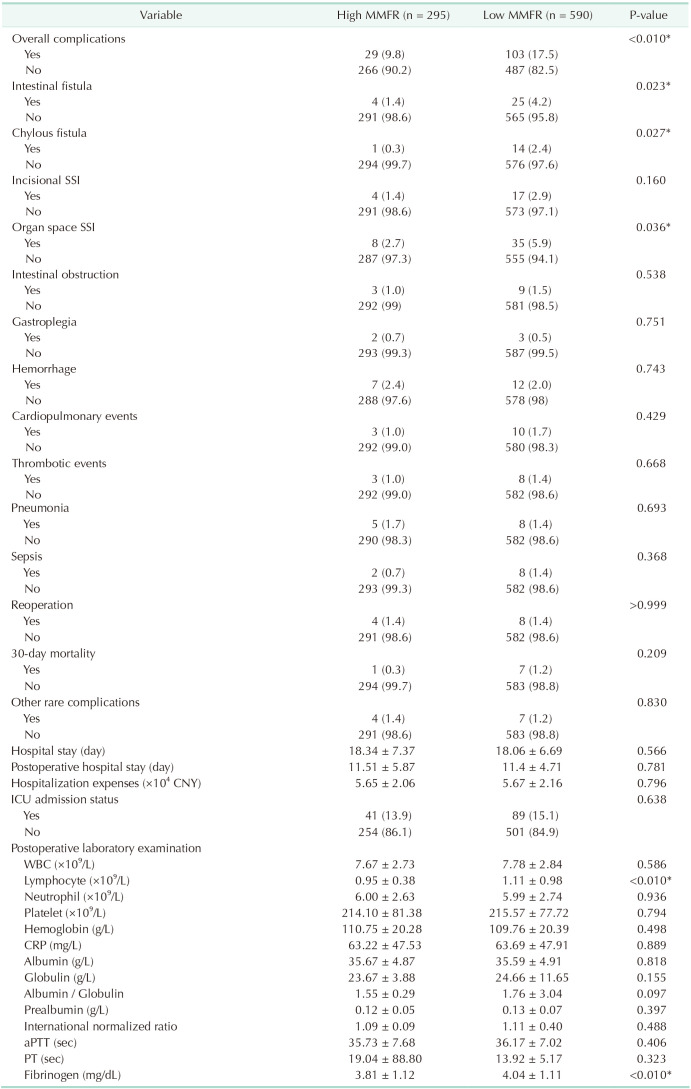
As shown in Table 3, postoperative levels of lymphocytes and fibrinogen were higher in the low MMFR group compared to the high MMFR group (P < 0.01). Overall complications in the low MMFR group were significantly higher than those in the high MMFR group (P < 0.01). Furthermore, the low MMFR group had a significantly higher incidence of intestinal fistula, chylous fistula, and organ space SSI compared to the high MMFR group (P = 0.023, P = 0.027, and P = 0.036, respectively).
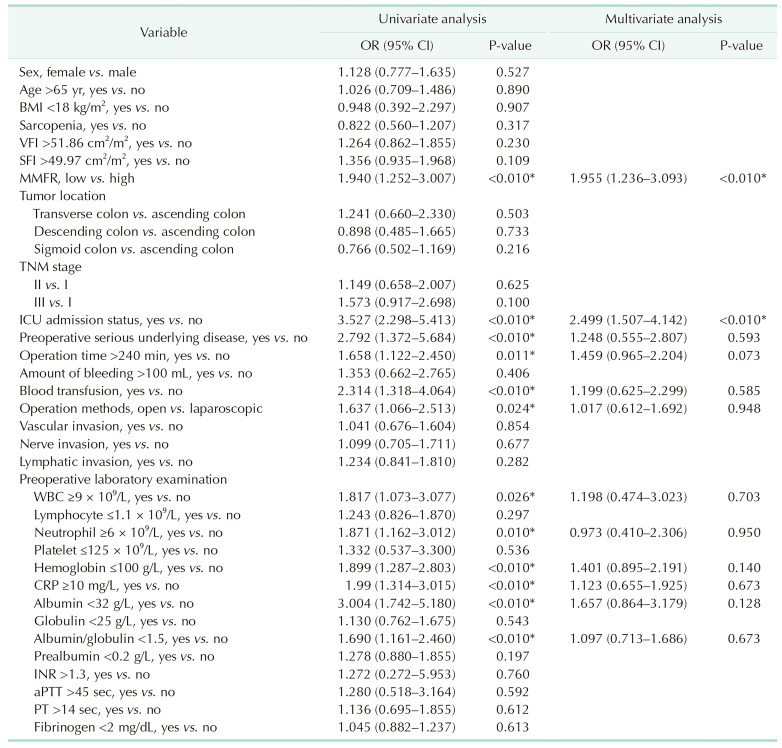
Univariate logistic regression analysis was conducted to determine the risk factors associated with overall complications. The factors that showed a significant association with overall complications were low MMFR (OR, 1.940; 95% CI, 1.252–3.007; P < 0.01), ICU admission (OR, 3.527; 95% CI, 2.298–5.413; P < 0.01), preoperative serious underlying disease (OR, 2.792; 95% CI, 1.372–5.684; P < 0.01), operation time ≥240 minutes (OR, 1.658; 95% CI, 1.122–2.450; P = 0.011), blood transfusion (OR, 2.314; 95% CI, 1.318–4.064; P < 0.01), operation methods (open vs. laparoscopic) (OR, 1.637; 95% CI, 1.066–2.513; P = 0.024), preoperative level of WBC ≥9 × 109/L (OR, 1.817; 95% CI, 1.073–3.077; P = 0.026), neutrophil ≥6 × 109/L (OR, 1.871; 95% CI, 1.162–3.012; P = 0.01), hemoglobin ≤100 g/L (OR, 1.899; 95% CI, 1.287–2.803; P < 0.01), CRP ≥10 mg/L (OR, 1.990; 95% CI, 1.314–3.015; P < 0.01), albumin <32 g/L (OR, 3.004; 95% CI, 1.742–5.180; P < 0.01), and albumin-globulin ratio <1.5 (OR, 1.690; 95% CI, 1.161–2.460; P < 0.01). Multivariate logistic regression analysis revealed that low MMFR (OR, 1.955; 95% CI, 1.236–3.093; P < 0.01) and ICU admission (OR, 2.499; 95% CI, 1.507–4.142; P < 0.01) were independently associated with overall complications (Table 4).
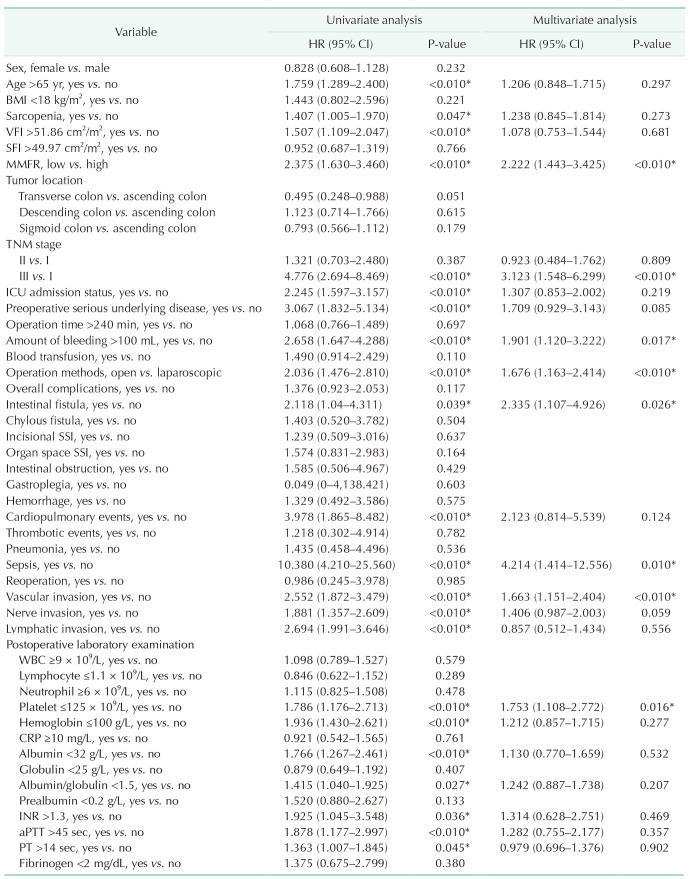
Univariate Cox regression analysis was performed to assess the factors affecting prognosis. The factors that showed a significant association with prognosis were age >65 years (HR, 1.759; 95% CI, 1.289–2.400; P < 0.01), sarcopenia (HR, 1.407; 95% CI, 1.005–1.970; P = 0.047), visceral fat index >51.86 cm2/m2 (HR, 1.507; 95% CI, 1.109–2.047; P < 0.01), low MMFR (HR, 2.375; 95% CI, 1.630–3.460; P < 0.01), TNM stage (III vs. I) (HR, 4.776; 95% CI, 2.694–8.469; P < 0.01), ICU admission (HR, 2.245; 95% CI, 1.597–3.157; P < 0.01), preoperative serious underlying disease (HR, 3.067; 95% CI, 1.832–5.134; P < 0.01), amount of bleeding ≥100 mL (HR, 2.658; 95% CI, 1.647–4.288; P < 0.01), operation methods (open vs. laparoscopic) (HR, 2.036; 95% CI, 1.476–2.810; P < 0.01), intestinal fistula (HR, 2.118; 95% CI, 1.040–4.311; P = 0.039), cardiopulmonary events (HR, 3.978; 95% CI, 1.865–8.482; P < 0.01), sepsis (HR, 10.380; 95% CI, 4.210–25.560; P < 0.01), vascular invasion (HR, 2.552; 95% CI, 1.872–3.479; P < 0.01), nerve invasion (HR, 1.881; 95% CI, 1.357–2.609; P < 0.01), lymphatic invasion (HR, 2.694; 95% CI, 1.991–3.646; P < 0.01), postoperative level of platelet ≤125 × 109/L (HR, 1.786; 95% CI, 1.176–2.713; P < 0.01), hemoglobin ≤100 g/L (HR, 1.936; 95% CI, 1.430–2.621; P < 0.01), albumin <32 g/L (HR, 1.766; 95% CI, 1.267–2.461; P < 0.01), albumin-globulin ratio <1.5 (HR, 1.415; 95% CI, 1.040–1.925; P = 0.027), INR >1.3 (HR, 1.925; 95% CI, 1.045–3.548; P = 0.036), aPTT >45 seconds (HR, 1.878; 95% CI, 1.177–2.997; P < 0.01), and PT >14 seconds (HR, 1.363; 95% CI, 1.007–1.845; P = 0.045). Multivariate Cox regression analysis revealed that low MMFR (HR, 2.222; 95% CI, 1.443–3.425; P < 0.01), TNM stage (III vs. I) (HR, 3.123; 95% CI, 1.548–6.299; P < 0.01), amount of bleeding ≥100 mL (HR, 1.901; 95% CI, 1.120–3.222; P = 0.017), operation methods (open vs. laparoscopic) (HR, 1.676; 95% CI, 1.163–2.414; P < 0.01), intestinal fistula (HR, 2.335; 95% CI, 1.107–4.926; P = 0.026), sepsis (HR, 4.214; 95% CI, 1.414–12.556; P = 0.01), vascular invasion (HR, 1.663; 95% CI, 1.151–2.402; P < 0.01), and postoperative level of platelets ≤125 × 109/L (HR, 1.753; 95% CI, 1.108–2.772; P = 0.016) were independently associated with prognosis (Table 5).
During follow-up, patients with low MMFR showed no significant difference in overall survival rate compared to those with high MMFR in the TNM stage I and II (P = 0.159 and P = 0.068, respectively). Patients with low MMFR showed significantly lower overall survival rates compared to those with high MMFR in the TNM stage III (P = 0.01). The total patients with low MMFR showed significantly lower overall survival rates compared to those with high MMFR (P < 0.01) (Fig. 3).
This population-based retrospective cohort study examined the impact of various factors on the risk of low MMFR and investigated the role of MMFR in predicting postoperative complications and long-term survival in patients with stage I–III colon cancer. The study found that female sex, older age, high BMI, sarcopenia, and high TNM stage were associated with low MMFR. Additionally, the study revealed that low MMFR can lead to changes in surgical methods, longer surgical times, and an increased risk of lymph node metastasis. Importantly, the study also revealed that low MMFR was strongly associated with postoperative complications, such as intestinal fistula, chylous fistula, and organ space SSI. Low MMFR was identified as an independent risk factor for overall complications and long-term survival in patients with colon cancer. To our knowledge, this study is one of the few to systematically investigate the effect of MMFR on overall complications and long-term survival in patients with colon cancer.
In recent years, there has been significant attention given to the influence of body composition parameters, such as skeletal muscle and fat, on colon cancer. Some reports have shown that sarcopenia is associated with longer hospital stays and higher medical costs, leading to further muscle loss [13]. In nonmetastatic colon cancer, sarcopenia has been found to increase postoperative complications, mortality, and tumor outcomes [14]. However, it can be challenging to screen and manage nutrition in high-risk populations due to the masking effect of overweight and obesity on sarcopenia. This phenomenon may explain why BMI is not a reliable prognostic indicator, as it fails to reflect differences in fat and muscle mass distribution. Fat, as an important body component, can contribute to the occurrence and development of colon cancer by affecting insulin signaling, increasing inflammation from adipose tissue, and disrupting sex hormone metabolism [15]. The increased subcutaneous fat in males and visceral fat in females are associated with a higher risk of death in patients with stage I–III colon cancer [10]. The increased visceral fat is also associated with poorer surgical outcomes, including higher rates of postoperative complications, SSI, and anastomotic fistula [16]. However, another research found that obese BMI was associated with better survival than underweight BMI in patients with colon cancer [17]. In this study, we found that sarcopenia, high visceral fat, and low MMFR showed a significant association with prognosis by using univariate Cox regression analysis. However, low MMFR was an independent factor that predicts worse overall survival instead of sarcopenia and high visceral fat by using multivariate Cox regression analysis. Considering the individual impact of skeletal muscle or fat separately may overlook the masking effect of overweight and obesity on sarcopenia. To evaluate postoperative complications and prognosis of colon cancer, we proposed preoperative MMFR by combining muscle and fat.
Skeletal muscle mass decreases linearly with age, while fat mass gradually increases. Moreover, females have a peak fat mass of more than 8 kg higher than males at the same age. Differences in muscle fiber size, composition, and hormone levels (estrogen and testosterone) contribute to greater overall skeletal muscle mass in males compared to females [18]. A retrospective study also found that even after adjusting for height and weight, males had greater overall skeletal muscle mass than age-matched females [19]. These findings are consistent with our study, which revealed that older females tend to have lower MMFR. Furthermore, our study found a significant correlation between low MMFR and higher levels of BMI, as well as a higher proportion of patients with sarcopenia. Currently, BMI is widely used as the main diagnostic method for obesity, and it is generally believed that higher BMI levels indicate a higher level of fat mass.
Our study revealed a strong correlation between the TNM stage and MMFR, indicating that the incidence of low MMFR increases as the tumor stage grade advances. In the TNM staging system, lymph nodes play a crucial role and are considered significant predictors of disease-free survival and overall survival in patients with colon cancer. The complete removal of the primary tumor and local lymph nodes is widely recognized as the most critical treatment for colon cancer. Therefore, accurately predicting the status of precancerous lymph nodes and optimizing treatment strategies for colon cancer are imperative [20]. Extensive lymph node resection in patients suspected of having positive lymph nodes may effectively reduce recurrence rates and improve long-term survival. Our study found that patients with a low MMFR were more likely to experience cancer lymph node metastasis compared to those with a high MMFR. This suggests that patients with a low MMFR may require a more extensive lymph node removal procedure. Additionally, we observed a close association between MMFR and operation methods. Patients with a low MMFR often have increased abdominal adipose tissue, which poses challenges for surgeons during the procedure. The presence of excess adipose tissue necessitates operating on a larger mesentery, resulting in a blurred surgical field of view. Consequently, it is difficult to identify an adequate surgical plane and normal vasculature, leading to prolonged operation times.
Our study reveals that MMFR is an independent risk factor for overall complications. Low MMFR is closely linked to intestinal fistula, chylous fistula, and organ space SSI. Previous research has demonstrated that surgical complications are an independent risk factor associated with reduced overall survival and increased recurrence rates [21]. Inflammatory cytokines released by surgical complications, such as anastomotic leakage and abdominal abscess, are believed to play a significant role in tumor progression or metastasis, thereby exacerbating prognosis [22]. Postoperative intestinal fistulas in surgical patients occur due to leakage of intestinal contents caused by compromised integrity of the intestinal wall at the anastomotic site. This leads to longer postoperative recovery time, higher distant tumor recurrence rates, and adverse effects on overall survival and disease-free survival [23]. Studies have reported that the incidence of intestinal fistula after colon cancer surgery ranges from 8% to 11.3%, with approximately 12% of patients with colon cancer succumbing to intestinal fistula [24]. A meta-analysis indicates that obesity increases the risk of intestinal fistula [25], while sarcopenia is strongly associated with postoperative intestinal fistula in patients with left colon cancer [26]. Our results showed that low MMFR increases the risk of intestinal fistula. Chylous fistula is a challenging disease caused by a blocked or ruptured lymph gland, resulting in significant nutritional and immunological consequences due to the continuous loss of proteins and lymphocytes. Patients with postoperative organ space SSI often experience an increased inflammatory response, which can create an environment for the survival and progression of residual cancer cells or latent micrometastases [27]. This is believed to occur through the stimulation of the angiogenesis pathway. Interestingly, a recent study suggests that the combination of visceral obesity and sarcopenia may be a more accurate predictor of postoperative complications in colon cancer compared to visceral obesity alone [28]. By implementing nutritional intervention and physical training to reduce skeletal muscle loss and fat accumulation before surgery, we can potentially reduce the occurrence of severe complications.
The Kaplan-Meier and Cox regression analyses revealed a significant association between low MMFR and low long-term survival rate. The combination of sarcopenia and obesity can increase the risk of death and recurrence rates in patients with various cancers, including hepatocellular carcinoma, stomach cancer, and pancreatic cancer. However, previous studies on this topic have included patients with both sarcopenia and obesity in the case group, using different definitions of obesity, such as the 2 highest quintiles of body fat percentage, VFA >100 cm2, or BMI >30 kg/m2 [2930]. These variations in the definition of obesity may either diminish or enhance the predictive effect of obesity on prognosis. Different from these studies, we quantify muscle and fat through the evaluation of muscle and adipose tissue area at L3 with CT scans. This approach allowed us to explore the prediction of prognosis using the MMFR, which holds great significance for comparing patients with different body types. The low survival rate caused by low MMFR can be attributed to several factors. Firstly, improvements in low MMFR can result in better management of other diseases, including hypertension, diabetes, chronic heart failure, and chronic obstructive pulmonary disease, and the risk of mortality from these chronic diseases may be reduced. Secondly, the decline in immune system function and the creation of an inflammatory tumor microenvironment caused by low MMFR contribute to the occurrence and progression of cancer, thereby increasing the cancer mortality rate. Lastly, low MMFR can lead to poor tolerance of various treatments, such as surgery, chemotherapy, and radiation.
The aims of low MMFR treatment are fat mass loss, together with fat-free mass preservation and physical function improvement. Preoperative dietary intervention combining quality and quantity of protein, hypocaloric diet, omega-3 and vitamin D may improve muscle strength, preserve muscle mass, and reduce muscle fat infiltration. Meanwhile, a combination of resistance and aerobic exercise before surgery could also improve physical function and reduce frailty.
The study has several limitations that should be considered. Firstly, it was a retrospective study conducted at a single center. Although a large number of patients were included, it would be beneficial to conduct the multicenter prospective study to validate its applicability in clinical practice. Secondly, our assessment of MMFR was limited to a single point before surgery. Changes in MMFR throughout a patient’s cancer treatment cycle could have a more significant impact on prognosis. Thirdly, while we made efforts to collect data on potential confounding factors, there were some variables that were not included, such as eating habits, physical activity, smoking status, alcohol consumption, and family history of cancer. Lastly, we defined the highest tertiles as the critical value of MMFR due to the lack of comprehensive research on the optimal critical value. Since the ratio of sarcopenia was exactly 2:3 in this study, we defined low MMFR as the 2 lowest tertiles to compare whether MMFR was superior to sarcopenia in predicting overall survival and complications. Further studies are required to confirm and establish the ideal critical value of MMFR.
In conclusion, the study indicated that MMFR was an independent risk factor for overall complications and survival in patients with stage I–III colon cancer. Meanwhile, an MMFR cutoff value of 0.585 for discriminating the prognosis, and the clinical effectiveness of this value was demonstrated. Due to the simultaneous consideration of the impacts of skeletal muscle and fat, MMFR may be a more reliable predictor of prognosis compared to sarcopenia in colon cancer patients. Considering the individual impact of skeletal muscle or fat separately may overlook the masking effect of overweight and obesity on sarcopenia. A hypocaloric protein-enriched diet and resistance and aerobic exercise before surgery to improve low MMFR could potentially enhance postoperative recovery and prognosis in patients with colon cancer.
Notes
References
1. Paik JH, Ryu CG, Hwang DY. Risk factors of recurrence in TNM stage I colorectal cancer. Ann Surg Treat Res. 2023; 104:281–287. PMID: 37179701.
2. Dai W, Mo S, Xiang W, Han L, Li Q, Wang R, et al. The critical role of tumor size in predicting prognosis for T1 colon cancer. Oncologist. 2020; 25:244–251. PMID: 32162825.
3. Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011; 12:489–495. PMID: 21296615.
4. Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019; 48:16–31. PMID: 30312372.
5. Dodson S, Baracos VE, Jatoi A, Evans WJ, Cella D, Dalton JT, et al. Muscle wasting in cancer cachexia: clinical implications, diagnosis, and emerging treatment strategies. Annu Rev Med. 2011; 62:265–279. PMID: 20731602.
6. Güç ZG, Altay C, Özgül HA, Ellidokuz H, Yavuzşen T. GNRI and CONUT scores: simple predictors of sarcopenia in metastatic colorectal cancer patients. Support Care Cancer. 2022; 30:7845–7852. PMID: 35716261.
7. Khan AI, Psutka SP, Patil DH, Hong G, Williams MA, Bilen MA, et al. Sarcopenia and systemic inflammation are associated with decreased survival after cytoreductive nephrectomy for metastatic renal cell carcinoma. Cancer. 2022; 128:2073–2084. PMID: 35285950.
8. Yamada K, Saito M, Ando M, Abe T, Mukoyama T, Agawa K, et al. Reduced number and immune dysfunction of CD4+ T cells in obesity accelerate colorectal cancer progression. Cells. 2022; 12:86. PMID: 36611881.
9. Kim JM, Chung E, Cho ES, Agawa K, Lee JH, Shin SJ, Lee HS, et al. Impact of subcutaneous and visceral fat adiposity in patients with colorectal cancer. Clin Nutr. 2021; 40:5631–5638. PMID: 34662848.
10. Brown JC, Caan BJ, Prado CM, Cespedes Feliciano EM, Xiao J, Kroenke CH, et al. The association of abdominal adiposity with mortality in patients with stage I–III colorectal cancer. J Natl Cancer Inst. 2020; 112:377–383. PMID: 31355882.
11. Yu PC, Hsu CC, Lee WJ, Liang CK, Chou MY, Lin MH, et al. Muscle-to-fat ratio identifies functional impairments and cardiometabolic risk and predicts outcomes: biomarkers of sarcopenic obesity. J Cachexia Sarcopenia Muscle. 2022; 13:368–376. PMID: 34866342.
12. Basile D, Rosati G, Bergamo F, Garattini SK, Banzi M, Zampino M, et al. Prognostic value of body mass index in stage II/III colon cancer: posthoc analysis from the TOSCA trial. Clin Colorectal Cancer. 2023; 22:190–198. PMID: 36935327.
13. Gani F, Buettner S, Margonis GA, Sasaki K, Wagner D, Kim Y, et al. Sarcopenia predicts costs among patients undergoing major abdominal operations. Surgery. 2016; 160:1162–1171. PMID: 27302103.
14. Zarinsefat A, Terjimanian MN, Sheetz KH, Stein IC, Mazurek AA, Waits SA, et al. Perioperative changes in trunk musculature and postoperative outcomes. J Surg Res. 2014; 191:106–112. PMID: 24750985.
15. Murphy N, Jenab M, Gunter MJ. Adiposity and gastrointestinal cancers: epidemiology, mechanisms and future directions. Nat Rev Gastroenterol Hepatol. 2018; 15:659–670. PMID: 29970888.
16. Watanabe J, Tatsumi K, Ota M, Suwa Y, Suzuki S, Watanabe A, et al. The impact of visceral obesity on surgical outcomes of laparoscopic surgery for colon cancer. Int J Colorectal Dis. 2014; 29:343–351. PMID: 24297037.
17. Lee S, Lee DH, Lee JH, Shin SJ, Lee HS, Park EJ, et al. Association of body mass index with survival in Asian patients with colorectal cancer. Cancer Res Treat. 2022; 54:860–872. PMID: 34665954.
18. Hawley SE, Bell ZW, Huang Y, Gibbs JC, Churchward-Venne TA. Evaluation of sex-based differences in resistance exercise training-induced changes in muscle mass, strength, and physical performance in healthy older (≥60 y) adults: a systematic review and meta-analysis. Ageing Res Rev. 2023; 91:102023. PMID: 37507092.
19. Janssen I, Heymsfield SB, Wang ZM, Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18–88 yr. J Appl Physiol (1985). 2000; 89:81–88. PMID: 10904038.
20. Kim HJ, Choi GS. Clinical implications of lymph node metastasis in colorectal cancer: current status and future perspectives. Ann Coloproctol. 2019; 35:109–117. PMID: 31288500.
21. Aoyama T, Oba K, Honda M, Sadahiro S, Hamada C, Mayanagi S, et al. Impact of postoperative complications on the colorectal cancer survival and recurrence: analyses of pooled individual patients’ data from three large phase III randomized trials. Cancer Med. 2017; 6:1573–1580. PMID: 28639738.
22. Matsubara D, Arita T, Nakanishi M, Kuriu Y, Murayama Y, Kudou M, et al. The impact of postoperative inflammation on recurrence in patients with colorectal cancer. Int J Clin Oncol. 2020; 25:602–613. PMID: 31758273.
23. Mungo B, Papageorge CM, Stem M, Molena D, Lidor AO. The impact of operative approach on postoperative complications following colectomy for colon caner. World J Surg. 2017; 41:2143–2152. PMID: 28332057.
24. Arron MN, Greijdanus NG, Bastiaans S, Vissers PA, Verhoeven RH, Ten Broek RP, et al. Long-term oncological outcomes after colorectal anastomotic leakage: a retrospective Dutch population-based study. Ann Surg. 2022; 276:882–889. PMID: 35930021.
25. He J, He M, Tang JH, Wang XH. Anastomotic leak risk factors following colon cancer resection: a systematic review and meta-analysis. Langenbecks Arch Surg. 2023; 408:252. PMID: 37386211.
26. Li Q, An T, Wu J, Lu W, Wang Y, Li J, et al. The impact of sarcopenia on the outcome of patients with left-sided colon and rectal cancer after curative surgery. BMC Cancer. 2023; 23:640. PMID: 37430182.
27. Alonso S, Pascual M, Salvans S, Mayol X, Mojal S, Gil MJ, et al. Postoperative intra-abdominal infection and colorectal cancer recurrence: a prospective matched cohort study of inflammatory and angiogenic responses as mechanisms involved in this association. Eur J Surg Oncol. 2015; 41:208–214. PMID: 25468742.
28. Feng Z, Pang K, Tian M, Gu X, Lin H, Yang X, et al. Sarcobesity, but not visceral fat, is an independent risk factor for complications after radical resection of colorectal cancer. Front Nutr. 2023; 10:1126127. PMID: 37260520.
29. Kim J, Han SH, Kim HI. Detection of sarcopenic obesity and prediction of long-term survival in patients with gastric cancer using preoperative computed tomography and machine learning. J Surg Oncol. 2021; 124:1347–1355. PMID: 34490899.
30. Malietzis G, Currie AC, Athanasiou T, Johns N, Anyamene N, Glynne-Jones R, et al. Influence of body composition profile on outcomes following colorectal cancer surgery. Br J Surg. 2016; 103:572–580. PMID: 26994716.
Fig. 2
CT measurement of skeletal muscle area at the third lumbar vertebra (L3). (A) Sagittal plane, the middle section of L3 is located on the yellow line. (B) Coronal plane, the middle section of L3 is located on the yellow line. (C) Horizontal cross section at L3. (D) Subcutaneous fat (blue), abdominal fat (yellow), and skeletal muscle (red).
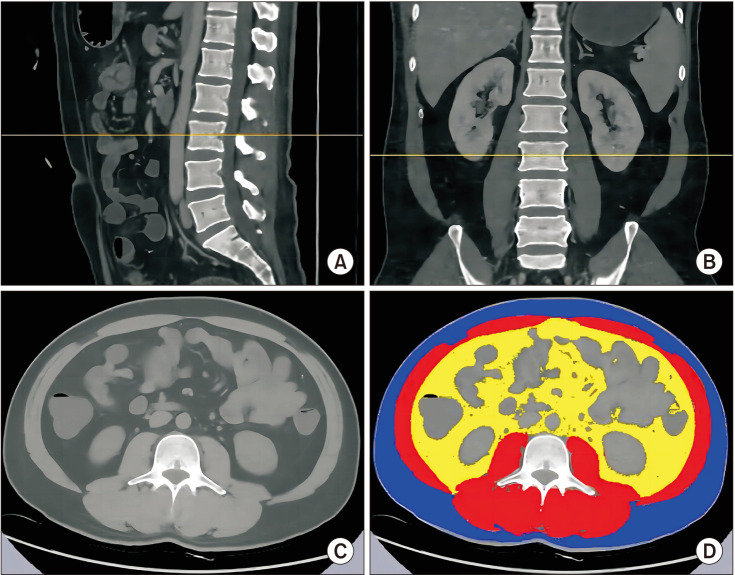
Fig. 3
Overall survival according to muscle mass-to-fat ratio (MMFR). (A) Patients with the TNM stage I. (B) Patients with the TNM stage II. (C) Patients with the TNM stage III. (D) Patients with all stages.
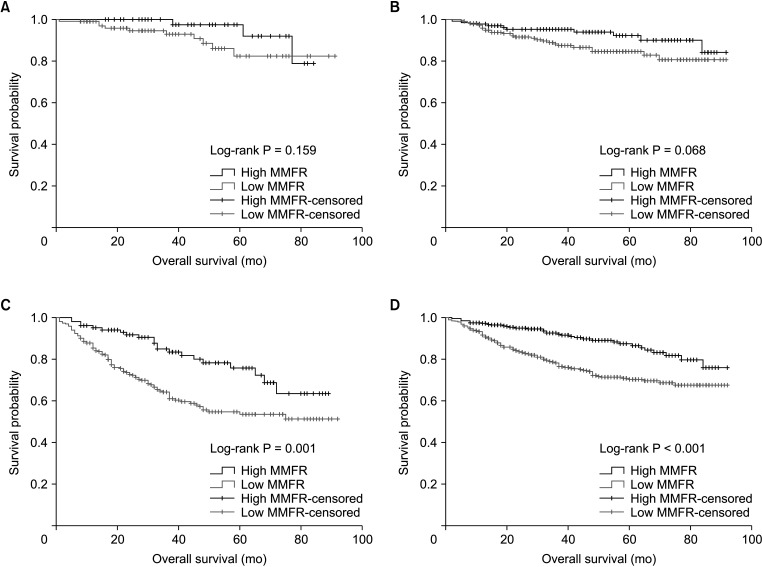
Table 1
Patients’ demographic and clinical characteristics according to muscle mass-to-fat ratio (MMFR)
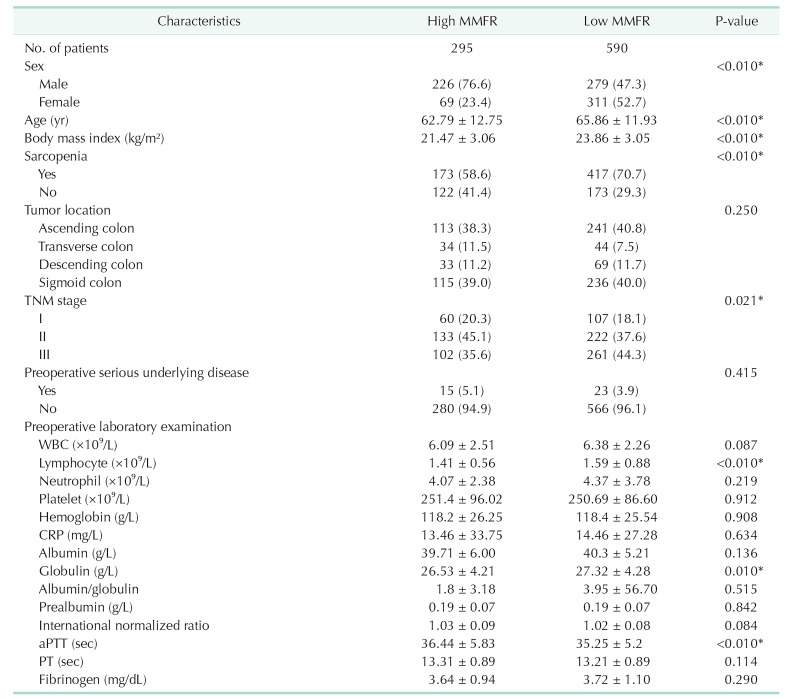
Table 4
Univariate and multivariate logistic regression analysis of risk factors for postoperative overall complications




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download