Abstract
Purpose
Laparoscopic right hemicolectomy is the standard surgical approach for treatment of right-sided colonic neoplasms. Although performed within a strict Enhanced Recovery After Surgery (ERAS) program, patients still develop postoperative ileus. The aim of this study was to describe the factors responsible for postoperative ileus after right hemicolectomy in a patient population with over 80% ERAS adherence.
Methods
In this retrospective study, we analyzed 499 consecutive patients undergoing elective right-sided colectomy for neoplastic disease in a single high-volume center. All patients followed an updated ERAS program.
Results
The overall median ERAS adherence was 80%. Patients with ≥ 80% adherence (n = 271) were included in further analysis. Their median ERAS adherence was 88.9% (interquartile range, 80–90; range, 80–100). Twenty-four of 271 patients (8.9%) developed postoperative ileus. A univariate regression analysis revealed carcinoma situated in the transverse colon, duration of operation over 200 minutes, and opiate consumption over 10 mg on the second postoperative day (POD) to be associated with a significantly higher risk of postoperative ileus. Multivariate regression analysis revealed that duration of surgery over 200 minutes (odds ratio [OR], 2.4; 95% confidence interval [CI], 1.0–5.8; P = 0.045) and opiate consumption over 10 mg on POD 2 (OR, 4.8; 95% CI, 1.6–14.3; P = 0.005) independently predict a higher risk for postoperative ileus. The median length of hospital stay was significantly longer in patients with postoperative ileus (8 days vs. 3 days, P < 0.001). None of the 271 patients died during a 30-day follow-up.
Enhanced Recovery After Surgery (ERAS) programs have greatly improved short-term surgical outcomes, length of hospital stay, and oncological survival in patients undergoing colon resection for neoplastic disease [1]. The cornerstones of an ERAS program are to preoperatively inform the patients adequately, prehabilitate before the surgical procedure, encourage early postoperative peroral feeding, and provide adequate and multimodal pain management with the idea of minimizing the use of opiates. All these main points are incorporated into a pathway consisting of individualized functions covering the patient’s treatment from the preoperative evaluation until discharge from the hospital after surgery [2].
Laparoscopic right hemicolectomy is the standard surgical procedure for neoplasms in the cecum, ascending colon, hepatic flexure, and proximal transverse colon [3]. Although ERAS is widely accepted and popularized, postoperative ileus remains a frequent complication after right hemicolectomy. The rate of postoperative ileus has been reported to remain as high as 19% in colorectal centers performing ERAS [4]. Furthermore, gradually rising ERAS adherence was concluded to have no effect on the incidence of postoperative ileus in a recent multicenter study [5]. Thus, the aim of our study was to clarify the factors associated with postoperative ileus in a group of strictly ERAS-adherent patients undergoing elective laparoscopic right hemicolectomy for neoplastic disease.
This study protocol was reviewed and approved by the Institutional Review Board of Helsinki University Hospital (No. HUS/115/2020) and this study adhered to the Declaration of Helsinki and the International Conference on the Harmonization of Good Clinical Practice. Finnish law allows for the use of medical records in medical research without necessitating the patient’s consent. The requirement for informed consent was waived in accordance with national guidelines by the Institutional Review Board of Helsinki University Hospital because of the retrospective nature of the study.
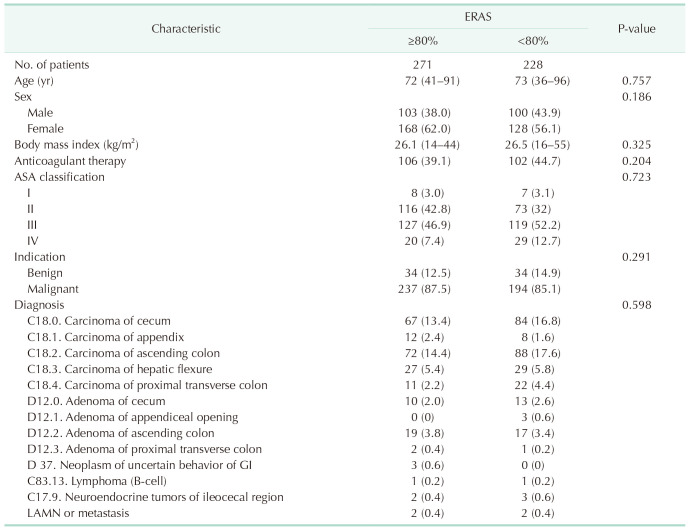
A single-center, retrospective study was carried out including all consecutive patients undergoing elective laparoscopic right hemicolectomy or extended right colectomy for malignant or benign neoplastic disease between January 2017 and December 2019. The exclusion criteria were emergency surgery, open surgery, active Crohn disease, and age under 18 years. A total of 499 patients were included. Of these, all patients with >80% ERAS adherence (n = 271) were included in further analysis. Results of patients with <80% ERAS adherence are shown as a comparison.
All the patients were treated according to our ERAS program optimized for elective colonic surgery. Our ERAS program was developed based on the ERAS Society guidelines for elective colorectal surgery [6].
Preoperatively, all elective patients meet a multidisciplinary team of a surgeon, a colorectal nurse, and an anesthesiologist in a predefined order. Detailed information on the planned surgical procedure is given. One month’s abstinence from alcohol and smoking is recommended. The nurse supervises the peroral intake on the day of surgery: clear fluids are allowed up to 2 hours, and solids up to 6 hours before the induction of anesthesia [7]. A preoperative carbohydrate drink is advised to be taken 2 hours prior to hospital arrival [89]. Medication is optimized (most importantly, peroral anticoagulants are paused and replaced) and prophylaxis against thrombosis is planned [89]. Compression stockings are measured to fit well. All instructions are given in both verbal and written forms.
A standardized anesthesia protocol is followed. Premedication is given only as an exception. Standard antimicrobial and postoperative nausea and vomiting (PONV) prophylaxes are administered [1011]. Cefuroxime prophylaxis is repeated if surgery is prolonged (>3 hours) and in case of extensive (>500 mL) bleeding [12]. The patient is kept normothermic and fluid balance is monitored with a restricted goal [1314]. Purely ropivacaine-based transversus abdominis plane (TAP) block, or rarely thoracic epidural analgesia (TEA), is preferably applied preoperatively or at the beginning of surgery for perioperative analgesia [15]. Paracetamol and nonsteroidal anti-inflammatory drugs (NSAIDs) (when no contraindications) are routinely used postoperatively. In the recovery room, patients are encouraged to drink 100–200 mL of fluids and are mobilized to sitting or standing. The time in the recovery room is kept reasonably short so the patient may benefit sooner from the better facilities for mobilization in the ward.
In the ward, oral fluids and supplements are given from the day of surgery [16]. Solid food is offered from the postoperative day (POD) 1 onwards [17]. Total fluid intake is kept at 20–30 mL/kg per day and intravenous (IV) administration is used if needed. Low molecular weight heparin is administered. With multimodal pain management, opioid use is kept low. Early mobilization is encouraged with the aim of spending a minimum of 6 hours out of bed from POD 1, with a physiotherapist being consulted when needed [18]. Urinary and epidural catheters are removed on POD 1 and 2, respectively [19].
The criteria for safe discharge are adequately controlled pain (oral analgesics), independent mobilization, normal diet (soup/solid), passage of air/stool and urine, and normothermia.
A standard laparoscopic procedure for right hemicolectomy or extended right colectomy was performed. Patients with malignant diagnoses were treated according to oncological principles. In the group of patients undergoing handsewn extracorporeal anastomosis (ECA), the resected bowel was extracted through a 5–7 cm incision in the upper right quadrant (or upper left in case of extended right colectomy). A plastic wound protector (Alexis, Applied Medical) was used. The anastomosis was performed end-to-end with 4-0 absorbable monofilament continuous suturing. In the stapled ECA group, the bowel was similarly extracted, and the anastomosis was performed with a GIA linear stapler (Medtronic), preferably side-to-side in an isoperistaltic fashion. The enterocolotomy was closed with 4-0 or 3-0 polydioxanone. For the patients undergoing intracorporeal anastomosis (ICA), the anastomosis was performed side-to-side in an isoperistaltic fashion intracorporeally using The Signia stapling system by Medtronic. The enterocolotomy was closed with a 3-0 V-loc (Medtronic) continuous double-layer suturing technique. Afterward, the resected bowel was extracted from a mini-Pfannenstiel opening. The fascial level of the extraction wound was closed with 2-0 absorbable monofilament continuous suturing. As part of our normal ERAS protocol, all the patients received prophylactic antibiotics within 1 hour before the surgical incision. Considering the possible risk of perioperative contamination in performing ICA, the ICA group received 3 doses of antibiotics postoperatively (a combination of cefuroxime and metronidazole).
In this study, all the patients were operated on or assisted and supervised by 1 or 2 experienced colorectal surgeons. Residents under the supervision and assistance of an experienced consultant performed only 7.0% of all the operations (19 of 271), in all of which a handsewn ECA was performed.
The incidence and predictive factors for postoperative ileus were analyzed as the primary outcome. Postoperative ileus was defined as abdominal distention with PONV from POD 2, a clinical or radiological confirmation of ileus, and an inability to tolerate oral intake from POD 2. Most of the patients were treated with a nasogastric tube. Additionally, the time to the first bowel movement after surgery was recorded and reported.
Other short-term postoperative complications (including the rate of anastomotic leakages, reoperations, and postoperative infections) and the length of hospital stay were analyzed as secondary outcomes. All complications were recorded using the upgraded Clavien-Dindo classification for colorectal surgery [20]. The Comprehensive Complication Index within 30 days after surgery was calculated as the sum of all complications, weighted for severity. The postoperative care, observations, and patient discharge were conducted according to the department’s ERAS protocol. Over 80% ERAS adherence was demanded for the patient to be analyzed further. Postoperative pain was monitored using visual analog scale scores from 0 to 10 and the consumption of pain medication was recorded in the patient’s medical record.
Overall, data gathered from the electronic patient records included sex, age, diagnosis, body mass index (BMI), American Society of Anesthesiologist (ASA) classification, Chalson comorbidity index score (CCI-score), anticoagulant treatment, preoperative hemoglobin level, preoperative albumin level, IV iron infusions, preoperative bowel preparation, preoperative antibiotic administration, preoperative medication, type of anastomosis, conversion to open surgery, the operating staff, TEA, TAP block, PONV prophylaxis, drainage, nasogastric tube, normothermia, IV fluids on the day of surgery, urinary catheter (the length kept in place), antithrombosis, postoperative mobilization, postoperative po intake (fluids, solids), bowel function recovery (air, solid stool), C-reactive protein (CRP) level on POD 2 and 3, analgesic regimen (paracetamol, NSAIDs, oxycodone), complications, length of hospital stay, reoperations, readmissions, adjuvant therapy, 30-day mortality.
Statistical analyses were performed using IBM SPSS Statistics for Windows (ver. 29.0, IBM Corp.). Continuous variables with normal distribution (age, BMI, intraoperative bleeding, duration of operation) are expressed as means with standard deviation and were compared using the one-way analysis of variance. Continuous variables with non-normal distribution (ERAS adherence, amount of postoperative opioids, first bowel movement, length of hospital stay, and CCI-score) are expressed in medians with interquartile range and compared with nonparametric tests. Categorical variables (anastomotic dehiscence, ileus, reoperation, 30-day mortality, readmission) were analyzed by the Pearson chi-square test and Fisher exact test. Univariate and multivariate logistic regression analyses were performed to study the predicting factors for postoperative ileus. The enter method was used in the multivariate analysis. The statistical significance threshold was set at a 2-sided α of 0.05.
The data of 580 patients were collected in total. Patients with nonneoplastic diagnoses or patients who underwent procedures other than right hemicolectomy (ileocecal resection or caecum resection) were excluded. Of the remaining 499 patients, all of those with ≥80% ERAS adherence (n = 271) were included in further analysis. The median ERAS adherence among these 271 patients was 88.9% (interquartile range [IQR], 80%–90%; range, 80%–100%). The demographic characteristics are detailed in Table 1. Comparison is made to patients with under 80% ERAS adherence (n = 228).
The mean duration of operation was 175 minutes (range, 74–403 minutes) and the mean amount of intraoperative bleeding was 90 mL (range, 0–1,350 mL). The median need for postoperative opioids was 27 mg (IQR, 15–43 mg; range, 0–172 mg). The median first postoperative bowel movement was recorded at 2 days (IQR, 1–2 days; range, 0–4 days), and the median length of hospital stay was 4 days (IQR, 3–5 days; range, 2–28 days). These results are reported in detail in Table 2. The comparison was made to patients with under 80% ERAS adherence (n = 228). The difference was significant considering the need for postoperative opioids (P = 0.006) and the length of hospital stay (P = 0.007).
Of all the 271 patients with ≥80% ERAS adherence, 96 (35.4%) developed at least 1 complication. The CCI score was calculated for every patient. The median CCI score was 0 (IQR, 0–20.9; range, 0–47.7).
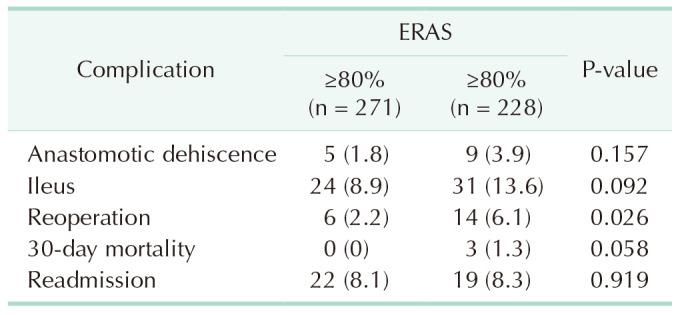
The rate of anastomotic dehiscence was 1.8%, and 2.2% of patients required reoperation. The 30-day readmission rate was 8.1% and 30-day mortality was 0%. These results are reported in detail in Table 3. The comparison was made to patients with under 80% ERAS adherence (n = 228). The difference was significant considering the rate of reoperations (P = 0.026).
A total of 24 of 271 patients (8.9%) developed postoperative ileus. No difference was observed in comparison to patients with under 80% ERAS adherence (Table 3).
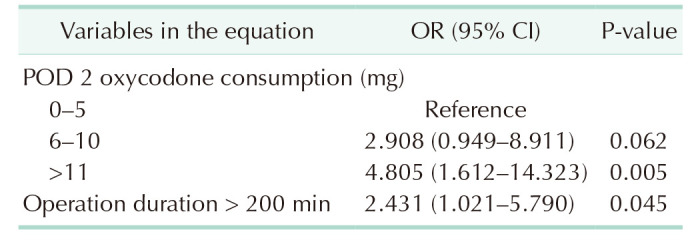
A univariate analysis was performed to study the factors associated with a risk of postoperative ileus in the 271 patients with ≥80% ERAS adherence (Table 4). This revealed that a duration of operation longer than 200 minutes and total postoperative oxycodone consumption of more than 10 mg on POD 2 significantly increase the risk of postoperative ileus. The anastomotic technique (ICA vs. handsewn ECA) did not associate with the risk of postoperative ileus.
A multivariate regression analysis was performed including all factors proven to be significant in the univariate analysis. This revealed duration of operation over 200 minutes (odds ratio [OR], 2.4; 95% confidence interval [CI], 1.0–5.8; P = 0.045) and oxycodone consumption over 10 mg on POD 2 (OR, 4.8; 95% CI, 1.6–14.3; P = 0.005) to independently predict a higher risk for postoperative ileus (Table 5).
A separate univariate analysis was performed for the inflammation marker CRP level on POD 3. Patients discharged before POD 2 were excluded (n = 29). We observed CRP-level higher than 120 on POD 3 to associate with the risk of postoperative ileus (OR, 3.3; 95% CI, 1.2–9.0; P = 0.021).
Altogether 6 of 271 patients (2.2%) required reoperation (Table 3). Four reoperations were performed due to anastomotic leakage. Of the remaining 2 patients, one had necrosis of the small bowel, and the other had small bowel herniation in a trocar wound. Both patients were treated with a segmental small bowel resection.
Altogether 22 of 271 patients (8.1%) were readmitted to the hospital during the first 30 PODs (Table 3). The most frequent cause of readmission was postoperative fever and/or infection (n = 9). Three patients had postoperative hemorrhage/anemia, 2 had postoperative ileus, 2 had urinary retention, 2 were dehydrated, 1 had atrial fibrillation, and 2 had abdominal pain and general postoperative weakness.
In comparison to many previous studies investigating postoperative complications, the present study was performed under a standardized and updated ERAS setting and concentrated on studying only patients with ERAS adherence ≥80% and undergoing an elective laparoscopic right-sided hemicolectomy with a neoplastic diagnosis. We report an 8.9% incidence of postoperative ileus in this high-ERAS adherent group. Despite appreciating the guideline of sparing opiates and managing perioperative pain multimodally (with 82.7% of patients having TAP blocks), we show even minor doses of postoperative opiates to predict a higher risk for ileus. Furthermore, a long duration of operation, which naturally represents a longer distraction from normal physiological functions and thus acts against the ERAS ideology, significantly increases the postoperative risk for ileus.
Postoperative ileus is defined as a temporary decrease in gastrointestinal motility after abdominal surgery. It is considered prolonged if the colonic dysmotility exceeds 72 hours. Postoperative ileus is more frequent after right hemicolectomy compared to left-sided resections [21]. A few possible reasons have been speculated. The “rectosigmoid brake” hypothesis suggests retrograde cyclic motor patterns (CMPs) occurring prominently after meals and limiting rectal filling contributing, thereby, to continence. These CMPs have been reported to become hyperactive after right hemicolectomy [22]. Also, the role of the ileocecal valve in peristalsis regulation and its removal in right hemicolectomy has been speculated to possibly disturb bowel function and cause bacterial translocation from the colon into the ileum. Furthermore, surgical trauma to the small intestine during right hemicolectomy can cause a stress reaction and paralysis [23]. The mini-invasive approach is proven to reduce surgical trauma. This fact has influenced the laparoscopic technique to become a standard approach in colorectal surgery, and it is strongly recommended in clinical guidelines such as those from the American Society of Colon and Rectal Surgeons and the Society of American Gastrointestinal and Endoscopic Surgeons [2].
The ileocolic anastomotic techniques have been studied for their potential role in the incidence of postoperative complications. ICA offers benefits by reducing mesenteric tension during the formation of the anastomosis and decreasing the length of incision needed for specimen extraction, both associated with earlier recovery. ICA has been reported to reduce pain, speed up postoperative bowel function recovery and shorten hospital stays [24]. However, a recent report by Cheong et al. [25] observed no significant difference in the overall perioperative and recovery outcomes, including postoperative complications. In this study, all 3 anastomotic techniques were used, and no effect was observed considering the rate of postoperative ileus.
The duration of a surgical procedure is associated with the risk of postoperative ileus. We corroborate this in strictly ERAS-adherent patients undergoing right-sided hemicolectomy. The explanations are multiple. One hypothesis is general anesthesia, which has several side effects; in particular, temporary impairment of gastrointestinal motility leading to postoperative ileus. The exact mechanism is still not well known. However, anesthetics are known to interact with receptors that involve visceral smooth muscle contractility induced by acetylcholine [26], which could affect bowel wall motility.
Duration of operation represents a partly modifiable factor. With increased surgical experience, the durations can be shortened. It has previously been reported that surgical experience plays an important role in both short- and long-term surgical outcomes and is associated with a decreased risk of both intra- and postoperative complications and a reduced length of hospital stay [27]. Within our setting, all operations were performed or assisted by an expert colorectal surgeon. Considering the nearly 9% rate of postoperative ileus, we support the recommendation that the extensive and radical oncologic colonic surgeries associated with a longer duration should especially be performed by a minimum of 2 senior colorectal surgeons.
Postoperative ileus is associated with significant financial consequences including increased healthcare costs. Therefore, efficient ERAS programs associated with decreased postoperative complications are of great healthcare and economic value [28]. The advantages of ERAS protocols have been studied in various surgical fields—for instance, patients following ERAS and undergoing laparoscopic gastrectomy have a significantly shorter time to first flatus and liquid intake after surgery than patients following traditional postoperative care [29]. Due to its common nature, the risk of postoperative ileus has been studied in general settings including patients undergoing different colonic resections, but the data for right-sided surgery, specifically, are scarce. Courtot et al. [30] reported male sex, epidural anesthesia, and the requirement for a blood transfusion as independent risk factors for postoperative ileus after right hemicolectomy. However, in our ERAS-adjusted patient population, with low intraoperative bleeding and widely used TAP block, we could not corroborate these findings.
This study has 2 main limitations: one is its inherent retrospective nature, and the other is that despite its high volume, it was conducted at a single center.
Reaching a high level of ERAS adherence does not prevent postoperative ileus from developing after an elective laparoscopic right hemicolectomy. However, we show an association between the duration of the operation, the dose of postoperative opiates, and the rate of postoperative ileus. Prolonged surgery, even minor doses of postoperative opiates, independently predicts a significantly higher risk of postoperative ileus in strictly ERAS-adherent patients after laparoscopic right hemicolectomy. These results highlight the undesired role of opiates after colon surgery and call for expertise to avoid unnecessary prolonging of surgery.
References
1. Viannay P, Hamy A, Jaouen R, Caroli-Bosc FX, Luel C, Vasseur S, et al. Does enhanced recovery improve the survival rates of patients 3 years after undergoing surgery to remove a tumor in the colon? Int J Colorectal Dis. 2019; 34:441–449. PMID: 30536115.
2. Carmichael JC, Keller DS, Baldini G, Bordeianou L, Weiss E, Lee L, et al. Clinical practice guidelines for enhanced recovery after colon and rectal surgery from the American Society of Colon and Rectal Surgeons and Society of American Gastrointestinal and Endoscopic Surgeons. Dis Colon Rectum. 2017; 60:761–784. PMID: 28682962.
3. Kim MK, Won DY, Lee JK, Kang WK, Kye BH, Cho HM, et al. Laparoscopic surgery for transverse colon cancer: short- and long-term outcomes in comparison with conventional open surgery. J Laparoendosc Adv Surg Tech A. 2015; 25:982–989. PMID: 26583447.
4. Alhashemi M, Fiore JF Jr, Safa N, Al Mahroos M, Mata J, Pecorelli N, et al. Incidence and predictors of prolonged postoperative ileus after colorectal surgery in the context of an enhanced recovery pathway. Surg Endosc. 2019; 33:2313–2322. PMID: 30334165.
5. Ripollés-Melchor J, Ramírez-Rodríguez JM, Casans-Francés R, Aldecoa C, Abad-Motos A, Logroño-Egea M, et al. Association between use of Enhanced Recovery After Surgery protocol and postoperative complications in colorectal surgery: the Postoperative Outcomes Within Enhanced Recovery After Surgery protocol (POWER) study. JAMA Surg. 2019; 154:725–736. PMID: 31066889.
6. Gustafsson UO, Scott MJ, Hubner M, Nygren J, Demartines N, Francis N, et al. Guidelines for perioperative care in elective colorectal surgery: Enhanced Recovery After Surgery (ERAS®) Society recommendations: 2018. World J Surg. 2019; 43:659–695. PMID: 30426190.
7. Forsmo HM, Pfeffer F, Rasdal A, Østgaard G, Mohn AC, Körner H, et al. Compliance with enhanced recovery after surgery criteria and preoperative and postoperative counselling reduces length of hospital stay in colorectal surgery: results of a randomized controlled trial. Colorectal Dis. 2016; 18:603–611. PMID: 27273854.
8. Rizvanović N, Nesek Adam V, Čaušević S, Dervišević S, Delibegović S. A randomised controlled study of preoperative oral carbohydrate loading versus fasting in patients undergoing colorectal surgery. Int J Colorectal Dis. 2019; 34:1551–1561. PMID: 31309323.
9. Kwon S, Meissner M, Symons R, Steele S, Thirlby R, Billingham R, et al. Perioperative pharmacologic prophylaxis for venous thromboembolism in colorectal surgery. J Am Coll Surg. 2011; 213:596–603. PMID: 21871823.
10. Weber WP, Mujagic E, Zwahlen M, Bundi M, Hoffmann H, Soysal SD, et al. Timing of surgical antimicrobial prophylaxis: a phase 3 randomised controlled trial. Lancet Infect Dis. 2017; 17:605–614. PMID: 28385346.
11. Feo CV, Sortini D, Ragazzi R, De Palma M, Liboni A. Randomized clinical trial of the effect of preoperative dexamethasone on nausea and vomiting after laparoscopic cholecystectomy. Br J Surg. 2006; 93:295–299. PMID: 16400707.
12. Bertschi D, Weber WP, Zeindler J, Stekhoven D, Mechera R, Salm L, et al. Antimicrobial prophylaxis redosing reduces surgical site infection risk in prolonged duration surgery irrespective of its timing. World J Surg. 2019; 43:2420–2425. PMID: 31292675.
13. Kurz A, Sessler DI, Lenhardt R. Study of Wound Infection and Temperature Group. Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. N Engl J Med. 1996; 334:1209–1215. PMID: 8606715.
14. Brandstrup B, Tønnesen H, Beier-Holgersen R, Hjortsø E, Ørding H, Lindorff-Larsen K, et al. Effects of intravenous fluid restriction on postoperative complications: comparison of two perioperative fluid regimens: a randomized assessor-blinded multicenter trial. Ann Surg. 2003; 238:641–648. PMID: 14578723.
15. Pedrazzani C, Menestrina N, Moro M, Brazzo G, Mantovani G, Polati E, et al. Local wound infiltration plus transversus abdominis plane (TAP) block versus local wound infiltration in laparoscopic colorectal surgery and ERAS program. Surg Endosc. 2016; 30:5117–5125. PMID: 27005290.
16. Smedley F, Bowling T, James M, Stokes E, Goodger C, O’Connor O, et al. Randomized clinical trial of the effects of preoperative and postoperative oral nutritional supplements on clinical course and cost of care. Br J Surg. 2004; 91:983–990. PMID: 15286958.
17. Lau C, Phillips E, Bresee C, Fleshner P. Early use of low residue diet is superior to clear liquid diet after elective colorectal surgery: a randomized controlled trial. Ann Surg. 2014; 260:641–649. PMID: 25203881.
18. Haines KJ, Skinner EH, Berney S. Austin Health POST Study Investigators. Association of postoperative pulmonary complications with delayed mobilisation following major abdominal surgery: an observational cohort study. Physiotherapy. 2013; 99:119–125. PMID: 23219632.
19. Zaouter C, Kaneva P, Carli F. Less urinary tract infection by earlier removal of bladder catheter in surgical patients receiving thoracic epidural analgesia. Reg Anesth Pain Med. 2009; 34:542–548. PMID: 19916208.
20. Katayama H, Kurokawa Y, Nakamura K, Ito H, Kanemitsu Y, Masuda N, et al. Extended Clavien-Dindo classification of surgical complications: Japan Clinical Oncology Group postoperative complications criteria. Surg Today. 2016; 46:668–685. PMID: 26289837.
21. Adamova Z, Filova M, Slovacek R. Factors determining postoperative ileus after surgery for colon cancer: comparison of right- and left-sided resections. Surg Technol Int. 2022; 40:140–146. PMID: 35166365.
22. Seo SH, Bissett I, O’Grady G. Variable gut function recovery after right vs. left colectomy may be due to rectosigmoid hyperactivity. Front Physiol. 2021; 12:635167. PMID: 33708140.
23. Boeckxstaens GE, de Jonge WJ. Neuroimmune mechanisms in postoperative ileus. Gut. 2009; 58:1300–1311. PMID: 19671558.
24. Kim S, Bae SU, Jeong WK, Baek SK, Son YG. Effect of intracorporeal anastomosis on postoperative ileus after laparoscopic right colectomy. Ann Surg Treat Res. 2023; 104:156–163. PMID: 36910563.
25. Cheong C, Kim NW, Lee HS, Kang J. Intracorporeal versus extracorporeal anastomosis in minimally invasive right hemicolectomy: systematic review and meta-analysis of randomized controlled trials. Ann Surg Treat Res. 2024; 106:1–10. PMID: 38205092.
26. Dryn D, Luo J, Melnyk M, Zholos A, Hu H. Inhalation anaesthetic isoflurane inhibits the muscarinic cation current and carbachol-induced gastrointestinal smooth muscle contractions. Eur J Pharmacol. 2018; 820:39–44. PMID: 29198958.
27. Sheetz KH, Norton EC, Birkmeyer JD, Dimick JB. Provider experience and the comparative safety of laparoscopic and open colectomy. Health Serv Res. 2017; 52:56–73. PMID: 26990210.
28. Traeger L, Koullouros M, Bedrikovetski S, Kroon HM, Thomas ML, Moore JW, et al. Cost of postoperative ileus following colorectal surgery: a cost analysis in the Australian public hospital setting. Colorectal Dis. 2022; 24:1416–1426. PMID: 35737846.
29. Tian YL, Cao SG, Liu XD, Li ZQ, Liu G, Zhang XQ, et al. Short- and long-term outcomes associated with enhanced recovery after surgery protocol vs conventional management in patients undergoing laparoscopic gastrectomy. World J Gastroenterol. 2020; 26:5646–5660. PMID: 33088158.
30. Courtot L, Le Roy B, Memeo R, Voron T, de Angelis N, Tabchouri N, et al. Risk factors for postoperative ileus following elective laparoscopic right colectomy: a retrospective multicentric study. Int J Colorectal Dis. 2018; 33:1373–1382. PMID: 29732465.




 PDF
PDF Citation
Citation Print
Print





 XML Download
XML Download