Abstract
Purpose
Preoperative localization plays an important role in secondary hyperparathyroidism (SHPT) surgery. The advantages of neck ultrasound (US) include high availability and low cost. However, the reported sensitivity of US is 54%–76%, and the reason for missed parathyroid glands (PGs) on US has been rarely addressed.
Methods
Fifty-four patients who were diagnosed with renal SHPT from September 2020 to March 2022 were included in this retrospective study. Preoperative localization included surgeon-oriented US and technetium 99m-sestamibi single-photon emission CT (SPECT)/CT.
Results
A total of 212 PGs were pathologically confirmed, resulting in a success rate of 96.2% (52 of 54). Using echo, 193 PGs (91.0%) were accurately localized, while 19 glands (9.0%) were not identified, including those in ectopic positions (n = 12, at thymus or intrathyroid or others), of small size (<1 cm, n = 6), or overlapping with an ipsilateral PG (n = 1). US accurately detected 4 PGs in 36 (66.7%) patients, while SPECT/CT localized 4 glands in 19 patients (35.2%). Although the number of US-detectable PGs was not associated with success rate, it showed a significant negative correlation with surgical time (rs = −0.459, P = 0.002).
Chronic kidney disease (CKD) is a major burden on global health. Patients with CKD stage III or higher often develop secondary parathyroid hyperplasia, caused by hyperphosphatemia and hypocalcemia [1]. Secondary hyperparathyroidism (SHPT) results in abnormal calcium deposition, osteoporosis, bone deformity, or fracture [2]. Surgical resection is the treatment option for severe SHPT when drug regimens fail. Several studies have confirmed that parathyroidectomy provides better quality of life, fewer major cardiovascular events, and lower risk of postoperative recurrence [345].
Currently, the success of parathyroidectomy relies on localization with imaging and surgeon experience [67]. However, the incidence of surgical failure, defined as persistent and recurrent SHPT after operation, is estimated to be 10%–30%, largely due to incomplete localization. Therefore, thorough localization by imaging might prevent surgical failure [689].
Ultrasound (US) localization for SHPT is commonly practiced in various centers due to its widespread availability, real-time imaging, lack of contrast requirement, absence of renal dysfunction risk, and cost-effectiveness [10]. Compared with normal parathyroid glands (PGs), 4-gland hyperplasia in SHPT may be more easily detected because of enlarged size. However, the preoperative imaging modality did not have high sensitivity in the detection of all the glands. The sensitivity of US localization ranges from 54% to 76% [111213]. The reported rate of US detection of 4 PGs ranges from 7.8% to 47.5% [1415].
Our objective aimed to assess the use of US and determine the reason for echo-detection failure. We must improve our technique accordingly to reduce surgical time and surgical failure rate.
This study was approved by the Institutional Review Board of Chang Gung Medical Foundation (No. 202300239B0). The need for informed consent from patients was waived because of the study’s retrospective design.
We retrospectively reviewed all SHPT patients who underwent total parathyroidectomy with or without autotransplantation between September 2020 and March 2022 in Kaohsiung Chang Gung Memorial Hospital, Taiwan. The indications for surgery were SHPT with any of the following: (1) age >18 years; (2) serum intact parathyroid hormone (iPTH) ≥800 pg/mL; (3) associated symptoms (pruritis, bone pain, fracture, and calciphylaxis); (4) refractory hypercalcemia (≥10.2 mg/dL) and/or hyperphosphatemia (≥6.0 mg/dL) despite pharmacologic therapy [16]. Initially, 63 SHPT patients were enrolled. Patients who had recurrent SHPT (n = 5), a history of thyroidectomy (n = 2), no preoperative US (n = 1), or were lost to follow up (n = 1) were excluded, thus leaving 54 patients for the final analysis (Fig. 1).
The PGs were determined to be eutopic or ectopic during operation [9]. An intrathyroidal PG was defined as a gland that was completely surrounded by thyroid tissue [17]. The normal range for iPTH is 15–76 pg/mL. Early surgical success was defined as the iPTH ≤ normal range on the postoperative day 5. Persistent SHPT was defined as the lowest iPTH exceeding the normal range within 6 months after parathyroidectomy. When initial postoperative iPTH was normal but returned to above the normal range more than 6 months after successful surgery, the patient was defined as recurrent SHPT [16].
Patient demographics, imaging reports, laboratory values, and operative and pathological results were collected. Laboratory values included serum calcium, phosphorus, and iPTH, which were checked on postoperative days 1 and 5 and 6 months thereafter [18]. The primary endpoint of the study was the US detection rate of PG and analysis of the reason for missed PGs. The secondary endpoint was the association of the number of image-detectable PGs with surgery success rate and operating time.
As part of our evaluation protocol, electrocardiography, echocardiography, and thallium scans were conducted before the operation. If positive or equivocal thallium scan results were obtained, patients were referred to cardiologists for percutaneous coronary intervention (PCI). For safety, parathyroid surgery was postponed for at least 2 months in patients who underwent PCI stenting followed by dual antiplatelet therapy. US and technetium 99m-sestamibi (MIBI) single-photon emission CT (SPECT)/CT were used for preoperative localization, and both were performed less than 1 month before surgery.
The US was performed by 1 of 3 experienced endocrine surgeons (Chan YC) with US diagnostic instruments (LOGIQ S8, GE Healthcare) and line array high-frequency probes (8–10 mHz). The typical parathyroid image showed an oval or multilobulated hypoechoic mass with a peripheral vascular arch and a parathyroid vascular pedicle (Fig. 2) [19]. Patients were placed in a supine position with their necks mildly extended. We searched 4 common gland locations, including the right upper, right lower, left upper, and left lower positions of the neck (Fig. 3A–D). The size of PG was measured in axial and transverse views. If the number of detected PGs was less than 4, the regions of the carotid sheath, thyrothymic trunk, and upper mediastinum were focused on to search for ectopic PGs. We did not perform a routine biopsy to confirm parathyroid hyperplasia. When an incidental thyroid nodule was detected on US, fine needle aspiration (FNA) was conducted according to current guidelines [20].
MIBI SPECT/CT imaging was performed on a Siemens Symbia T SPECT/CT gamma camera (Siemens Medical Solutions). After intravenous administration of 1,110 MBq (30 mCi) of MIBI via a lower limb vein, each patient underwent early-phase planar imaging and delayed phase planar imaging followed by SPECT/CT. The image was considered positive if it showed a focal area of increased uptake in the neck, mediastinum, or any potential ectopic site with either a progressive increase or a prolonged retention at the delayed phase.
US and parathyroidectomy were performed by the same surgical team. The results of US and SPECT/CT were read by surgeons at the time of surgery. Every patient underwent bilateral 4-gland neck exploration via a transverse cervical incision under general anesthesia. When 4 or more enlarged PGs were removed, 100 mg of the smallest gland was implanted into the subcutaneous layer of the limb. If fewer than 3 PGs were identified, no autotransplantation would take place due to risks of surgical failure. We performed routine bilateral transcervical thymectomy for the possibility of ectopic PGs. The location of PG was recorded during surgery (Fig. 3E) and each gland was classified as true positive, false positive, true negative, or false negative based on US findings and pathology. Additionally, the surgeon defined the ectopic location and cause of US-missed PGs intraoperatively. The size and weight of PGs were measured immediately after removal. Hemithyroidectomy or total thyroidectomy was conducted in some patients with a large nodular goiter (>2 cm), or a nodule suspected or confirmed malignancy via US or FNA. The removed PGs and/or thyroid were examined by pathologists for the final diagnosis. Postoperative follow-up was longer than 6 months to define SHPT recurrence or relapse.
Numerical data are expressed as the mean ± standard deviation and categorical data are expressed as rates or proportions. Logistic regression and Spearman correlation were used for statistical analysis. Results were considered significant if the P-value was <0.05. Statistical analysis was performed using IBM SPSS Statistics ver. 20.0 (IBM Corp.).
In all, 212 PGs of 54 uremic patients were removed with histological confirmation of parathyroid hyperplasia. The mean age of the patients was 58.7 ± 11.9 years. Male patients accounted for 61.1% (33 of 54) of this cohort. The mean duration of surgery was 83.5 ± 31.0 minutes (Table 1). Two patients (3.7%) were diagnosed with persistent SHPT after operation and no recurrent SHPT or permanent hypoparathyroidism after a mean follow-up period of 19.4 months.
There were 28 ectopic PGs (13.1%) in this study. The locations of these ectopic PGs were as follows: thymus (n = 15, 7.0%), intrathyroid (n = 3, 1.4%), and others (n = 10, 4.7%). Eleven patients underwent concurrent lobectomy (n = 5, 9.3%) or total thyroidectomy (n = 6, 11.1%) for nodular goiter (n = 7, 13.0%) or thyroid cancer (n = 4, 7.4%).
There were 2 surgical failures. We excised 4 extrathyroid nodules, but pathology proved that only 3 nodules were parathyroid hyperplasia and the other one was nodular goiter. The patient had persistent SHPT (postoperative iPTH, 261 pg/mL). In another case, we also excised 4 extrathyroid nodules, all of which were confirmed to be parathyroid hyperplasia. However, the postoperative iPTH was 665 pg/mL, therefore supernumerary PGs were suspected. The 2 patients did not undergo imaging evaluation after the first operation.
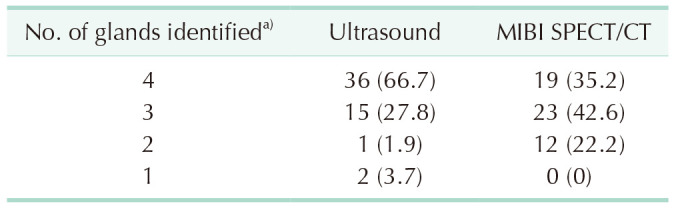
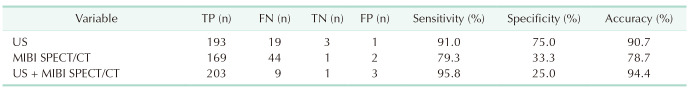
In Table 2, US showed all 4 PGs in 36 patients (66.7%), 3 PGs in 15 (27.8%), 2 PGs in 1 (1.9%), and only 1 PG in 2 (3.7%). SPECT/CT detected all 4 PGs in 19 patients (35.2%), 3 PGs in 23 (42.6%), and 2 PGs in 12 (22.2%). When analyzed by lesion number, the sensitivity of US, SPECT, and combination was 91.0% (193 of 212), 79.3% (169 of 212), and 95.8% (203 of 212), respectively. The specificity of US, SPECT, and combination was 75.0% (3 of 4), 33.3% (1 of 3), and 25.0% (1 of 4), respectively. The accuracy of US, SPECT, and combination was 90.7% (196 of 216), 78.7% (170 of 216), and 94.4% (204 of 216), respectively (Table 3).
Compared with intraoperative findings and final pathology, 1 gland on US and 2 glands on SPECT/CT were false positives (Table 3). Meanwhile, 19 PGs (9.0%) were not shown on US and were categorized as false negatives. Table 4 summarizes the reason for US-missed PGs. The most common cause was an ectopic position (n = 12, 3.2%), including thymus (n = 6, 2.8%) (Fig. 4A), intrathyroid (n = 1, 0.5%) (Fig. 4B), and others (located at mediastinum, retroesophageal, and retropharyngeal area; n = 5, 2.4%). The second most common cause was small size (n = 6, 2.8%), which all was less than 1 cm in maximal length and weighed less than 200 µg. One upper PG overlap with the ipsilateral lower PG (n = 1, 0.5%) (Fig. 4C) also led to a missed US cause.
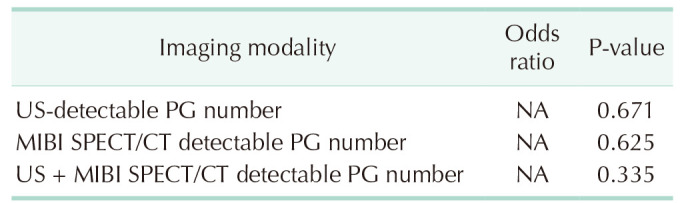
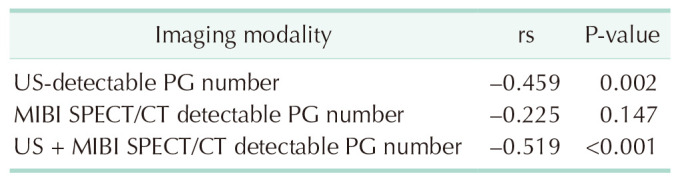
The analysis of the association between the number of US, SPECT/CT, o r US+SPECT/CT detected P Gs a nd t he surgical success rate showed no significance (P = 0.671, P = 0.625, and P = 0.335, respectively) (Table 5). The number of SPECT/CT detected PGs was not correlated with surgical time (rs = −0.225, P = 0.147), but the number of US- or US + SPECT/CT-detected PGs was negatively associated with surgical time (rs = −0.459, rs = −0.519 and P = 0.002, P < 0.001, respectively), as shown in Table 6.
Ultrasonography is one of the most common modalities for localization of hyperfunctioning PGs. However, several studies have noted that the sensitivity of US varies greatly, and the reason for missed gland has not been discussed in detail. In this study, we found that US for preoperative localization had 90% sensitivity and accuracy. Visualizing all 4 parathyroid hyperplasia can be achieved in 66.7% and 35.2% of SHPT patients by US and SPECT/CT, respectively. PGs located in an ectopic position or of a small size (<1 cm) might cause negative results on US. Furthermore, if we could completely map the hyperplastic glands, the surgical time could be reduced.
The ectopic position was the most common cause of US-missed localization. In our study, the incidence of ectopic glands was 13.2%, 42.9% of which could not be recognized on US. The study by Andrade et al. [15] corroborated these findings. They recruited 166 secondary and tertiary HPT patients. Thirteen percent of hyperfunctioning PGs were located in ectopic positions, and 61.5% of these ectopic PGs failed to be localized by US and MIBI scintigraphy. A study by Alkhalili et al. [14] concluded that half of ectopic PGs were not detected by US or MIBI. Several different definitions of ectopic glands have led to variations in the incidence. The most common ectopic sites included the thymus, retroesophageal, retropharyngeal, intrathyroid, mediastinum, and carotid sheath [91415]. Hyperfunctioning PGs within the mediastinum or in retroesophageal locations where bone and air, respectively, prevent visualization of deeper structures. Because missed PGs in ectopic positions were the leading cause of failed surgery, awareness of the disadvantages of US is important for surgical planning.
Small PGs (<1 cm) were determined to be a limitation for US detection. Périé et al. [19] found that false negative results on ultrasonography correlated with low gland weight. A small gland was considered equally as low weight. Although we found that not all small glands are undetectable on US, all the missed PGs that were in eutopic positions were smaller than 1 cm. In a prospective study of 1,000 consecutive patients with primary HPT, body mass index and gland size independently predicted the accurate detection of PGs by US and MIBI [21]. Furthermore, when the maximal length was smaller than 1 cm, more than half of PGs were undetectable [21]. Either in primary or SHPT, abnormal PGs less than 1 cm in length were associated with false negatives on US.
The overwhelming majority of individuals have 4 PGs, 2 superiors and 2 inferiors, whereas between 2.5% and 13.0% have supernumerary glands, and 3% have fewer than 4 glands [2223]. We did not find any supernumerary PGs, but there was 1 suspicious case due to persistent HPT after excision of 4 parathyroid hyperplasias. Our protocol for parathyroid US consisted of searching 4 common positions for PGs, and supernumerary glands would therefore be missed. Furthermore, surgical failure is mainly due to resecting not all the PGs, coming from the existence of ectopic or supernumerary PGs [122425]. To improve the surgical success rate, precise preoperative imaging localization is crucial. Andrade et al. [15] noted that US and MIBI could not detect supernumerary PGs. El-Sageer et al. [7] recommended that neck exploration was the most reliable method for localization than imaging modalities. However, from our experience, ectopic PGs in the thyroid, carotid sheath, or mediastinum were difficult to detect during the operation, and searching of the neck prolonged the operative time. Preoperative mapping for parathyroid surgery is rational and recommended.
This study has several limitations. First, MIBI SPECT/CT provided additional information for preoperative localization. Second, the judgment of US-missed PG was decided by endocrine surgeons who were not blinded. Third, 2 surgical failure cases with persistent HPT did not undergo imaging survey and reoperation until now. Additionally, concurrent thyroidectomy might increase the surgical success rate. Finally, the study was a retrospective design with a small sample size. More prospective studies with larger groups are needed.
Our findings demonstrated that US localization was accurate in 90% of our patients. Awareness of the limitations of US is important for surgeons searching for hyperplastic PGs, especially those situated in ectopic positions and small sizes. US provided a useful guide for localization and, therefore, shortened the operation time.
ACKNOWLEDGEMENTS
We thank the Biostatistics Center, Kaohsiung Chang Gung Memorial Hospital, for their statistics work.
Notes
References
1. Kumar R, Thompson JR. The regulation of parathyroid hormone secretion and synthesis. J Am Soc Nephrol. 2011; 22:216–224. PMID: 21164021.
2. Ketteler M, Block GA, Evenepoel P, Fukagawa M, Herzog CA, McCann L, et al. Executive summary of the 2017 KDIGO Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Guideline Update: what’s changed and why it matters. Kidney Int. 2017; 92:26–36. PMID: 28646995.
3. Komaba H, Taniguchi M, Wada A, Iseki K, Tsubakihara Y, Fukagawa M. Parathyroidectomy and survival among Japanese hemodialysis patients with secondary hyperparathyroidism. Kidney Int. 2015; 88:350–359. PMID: 25786097.
4. Apetrii M, Goldsmith D, Nistor I, Siriopol D, Voroneanu L, Scripcariu D, et al. Impact of surgical parathyroidectomy on chronic kidney disease-mineral and bone disorder (CKD-MBD): a systematic review and meta-analysis. PLoS One. 2017; 12:e0187025. PMID: 29107998.
5. Madorin C, Owen RP, Fraser WD, Pellitteri PK, Radbill B, Rinaldo A, et al. The surgical management of renal hyperparathyroidism. Eur Arch Otorhinolaryngol. 2012; 269:1565–1576. PMID: 22101574.
6. Steinl GK, Kuo JH. Surgical management of secondary hyperparathyroidism. Kidney Int Rep. 2020; 6:254–264. PMID: 33615051.
7. El-Sageer EM, Shehata AM, Khalaf M, El-Heeny AA. Neck exploration versus imaging localization of parathyroid in secondary hyperparathyroidism. Indian J Surg. 2019; 81:457–461.
8. Petranović Ovčariček P, Giovanella L, Hindie E, Huellner MW, Talbot JN, Verburg FA. An essential practice summary of the new EANM guidelines for parathyroid imaging. Q J Nucl Med Mol Imaging. 2022; 66:93–103. PMID: 35166093.
9. Reitz RJ 3rd, Dreimiller A, Khil A, Horwitz E, McHenry CR. Ectopic and supernumerary parathyroid glands in patients with refractory renal hyperparathyroidism. Surgery. 2021; 169:513–518. PMID: 32919783.
10. Sung JY. Parathyroid ultrasonography: the evolving role of the radiologist. Ultrasonography. 2015; 34:268–274. PMID: 25971897.
11. Mohammadi A, Moloudi F, Ghasemi-Rad M. Preoperative localization of parathyroid lesion: diagnostic usefulness of color doppler ultrasonography. Int J Clin Exp Med. 2012; 5:80–86. PMID: 22328952.
12. Hiramitsu T, Tomosugi T, Okada M, Futamura K, Tsujita M, Goto N, et al. Pre-operative localisation of the parathyroid glands in secondary hyperparathyroidism: a retrospective cohort study. Sci Rep. 2019; 9:14634. PMID: 31602011.
13. Zhang R, Zhang Z, Huang P, Li Z, Hu R, Zhang J, et al. Diagnostic performance of ultrasonography, dual-phase 99mTc-MIBI scintigraphy, early and delayed 99mTc-MIBI SPECT/CT in preoperative parathyroid gland localization in secondary hyperparathyroidism. BMC Med Imaging. 2020; 20:91. PMID: 32746794.
14. Alkhalili E, Tasci Y, Aksoy E, Aliyev S, Soundararajan S, Taskin E, et al. The utility of neck ultrasound and sestamibi scans in patients with secondary and tertiary hyperparathyroidism. World J Surg. 2015; 39:701–705. PMID: 25409841.
15. Andrade JS, Mangussi-Gomes JP, Rocha LA, Ohe MN, Rosano M, das Neves MC, et al. Localization of ectopic and supernumerary parathyroid glands in patients with secondary and tertiary hyperparathyroidism: surgical description and correlation with preoperative ultrasonography and Tc99m-Sestamibi scintigraphy. Braz J Otorhinolaryngol. 2014; 80:29–34. PMID: 24626889.
16. Tominaga Y. Surgical treatment of secondary hyperparathyroidism due to chronic kidney disease. Ups J Med Sci. 2006; 111:277–292. PMID: 17578795.
17. Phitayakorn R, McHenry CR. Incidence and location of ectopic abnormal parathyroid glands. A m J Surg. 2006; 191:418–423.
18. Chou YC, Chan YC, Chi SY, Chou FF. Being elderly is not a contraindication of parathyroidec tomy for renal hyperparathyroidism and chronic kidney disease: mineral and bone disorder. Asian J Surg. 2021; 44:321–328. PMID: 32891512.
19. Périé S, Fessi H, Tassart M, Younsi N, Poli I, St Guily JL, et al. Usefulness of combination of high-resolution ultrasonography and dual-phase dual-isotope iodine 123/technetium Tc 99m sestamibi scintigraphy for the preoperative localization of hyperplastic parathyroid glands in renal hyperparathyroidism. Am J Kidney Dis. 2005; 45:344–352. PMID: 15685513.
20. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016; 26:1–133. PMID: 26462967.
21. Berber E, Parikh RT, Ballem N, Garner CN, Milas M, Siperstein AE. Factors contributing to negative parathyroid localization: an analysis of 1000 patients. Surgery. 2008; 144:74–79. PMID: 18571587.
22. Akerström G, Malmaeus J, Bergström R. Surgical anatomy of human parathyroid glands. Surgery. 1984; 95:14–21. PMID: 6691181.
23. Nakatsuka K, Nishizawa Y, Ishimura E, Miki T, Hagiwara S, Morita A, et al. The fifth hyperfunctioning parathyroid gland in end-stage renal disease. Nephron. 1989; 51:140–142. PMID: 2915752.
24. Schneider R, Waldmann J, Ramaswamy A, Fernández ED, Bartsch DK, Schlosser K. Frequency of ectopic and supernumerary intrathymic parathyroid glands in patients with renal hyperparathyroidism: analysis of 461 patients undergoing initial parathyroidectomy with bilateral cervical thymectomy. World J Surg. 2011; 35:1260–1265. PMID: 21479685.
25. Taterra D, Wong LM, Vikse J, Sanna B, Pękala P, Walocha J, et al. The prevalence and anatomy of parathyroid glands: a meta-analysis with implications for parathyroid surgery. Langenbecks Arch Surg. 2019; 404:63–70. PMID: 30762091.
Fig. 1
Flowchart of screening for eligible cases. SHPT, secondary hyperparathyroidism; MIBI, technetium 99m-sestamibi; SPECT, single-photon emission CT.
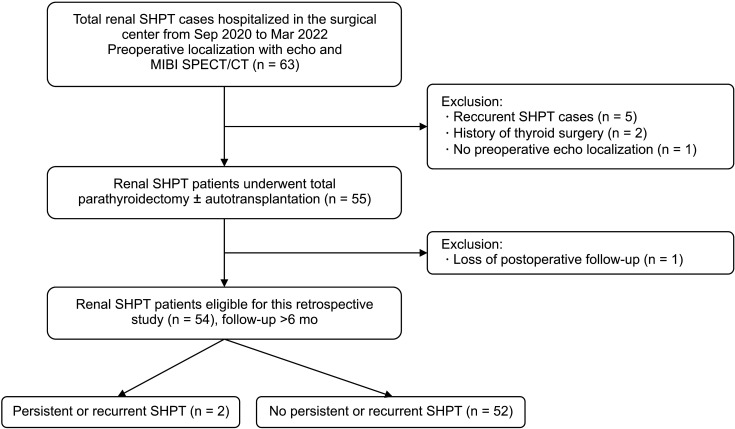
Fig. 2
Parathyroid hyperplasia was shown on ultrasound in transversal (A) and longitudinal (B) views. Typical characteristics include homogenous hypoechogenicity, oval or multilobulated shape, and peripheral vascularity.
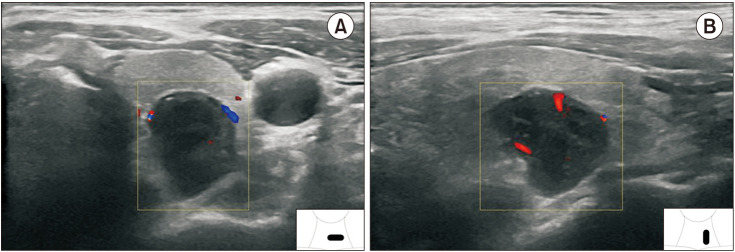
Fig. 3
Ultrasound in transverse views of an ESRD patient (A: right upper parathyroid gland [RUP], B: right lower parathyroid gland [RLP], C: left upper parathyroid gland [LUP], and D: left lower parathyroid gland [LLP]) showing secondary parathyroid hyperplasia compared with intraoperative findings (E).
J, internal jugular vein; C, carotid artery; NG, nodular goiter; T, trachea; TH, thymus.
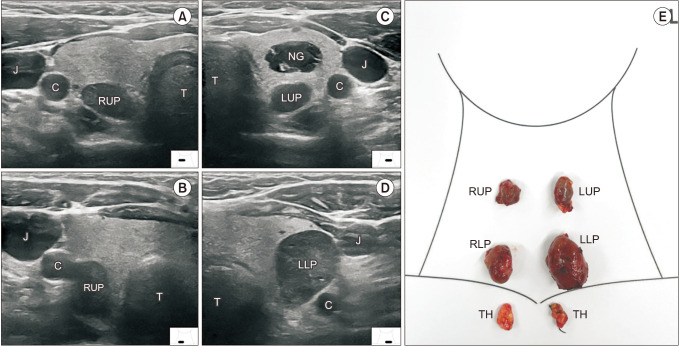
Fig. 4
Causes of ultrasound-missed parathyroid hyperplasia. (A) Ectopic bilateral lower parathyroid glands in the thymus. (B) The left upper parathyroid gland (LUP) was misdiagnosed as part of the thyroid nodule (NG), compared to the intraoperative findings (right). (C) The right upper parathyroid gland (RUP) overlapped with the right lower one (RLP), compared to the intraoperative finding (right). C, carotid artery; NG, nodular goiter; TH, thymus.
Table 1
Basic characteristics of the 54 ESRD patients with SHPT
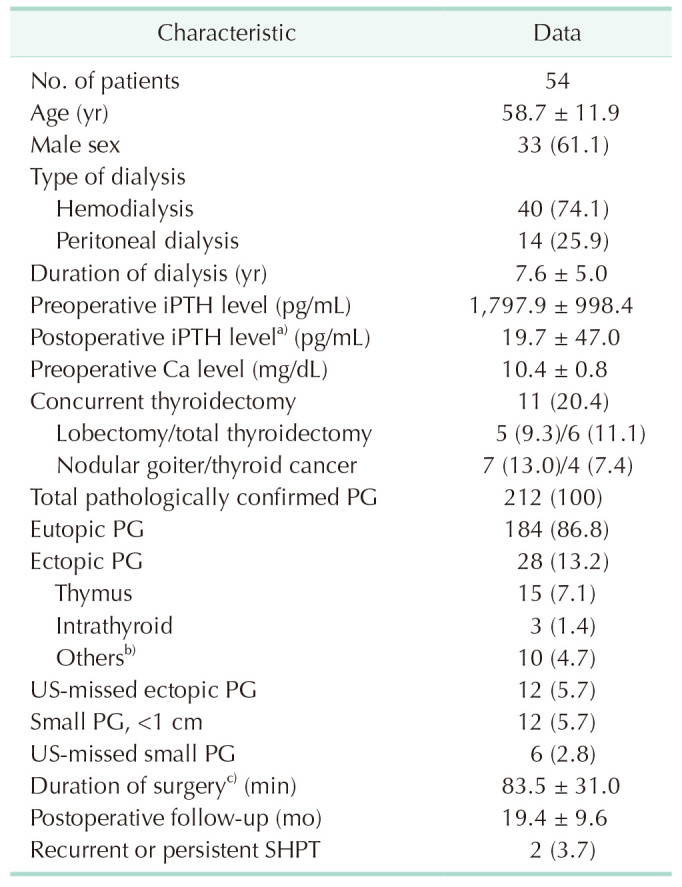
Values are expressed as number only, means ± standard deviation, or number (%).
ESRD; end-stage renal disease; SHPT, secondary hyperparathyroidism; iPTH, intact parathyroid hormone; PG, parathyroid gland.
a)Postoperative day 3. b)PGs in mediastinum, retroesophageal, retropharyngeal locations. c)Exclude patients who underwent concurrent thyroidectomy.




 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download