Abstract
Purpose
This study was performed to compare the therapeutic efficacy of endoscopic surgery and open surgery and their effects on postoperative blood coagulation state in patients with thyroid cancer, and to provide evidence for the prevention measurement of thrombosis in the perioperative period.
Methods
One hundred patients with thyroid cancer who received treatment in our hospital from January 2021 to December 2021, were randomly divided into an endoscopic group and an open surgery group, with 50 patients in each group. The patients in the open surgery group were treated by traditional open surgery, while patients in the endoscopic group accepted endoscopic surgery. The clinically therapeutic effect and blood coagulation of the 2 groups were compared.
Results
Intraoperative blood loss and length of hospital stay were lower, and operative time was longer in the endoscopic group than in the open surgery group (P < 0.05). The 24-hour postoperative fibrinogen and D-dimer levels were higher in both groups than in the preoperative period, while PT was shorter (P < 0.05). There were no significant differences in postoperative complications and follow-up between the 2 groups (P > 0.05), but the incidence of complications, postoperative metastases, and thrombosis was relatively low in the endoscopic group.
Thyroid cancer is the most common malignancy of the head and neck. Between 1990 and 2013, the age-standardized incidence of thyroid cancer increased by 20% globally, with the relative increase in low-income countries (33%) being significantly higher than that in high-income countries (19%) [12]. In 2022, the National Cancer Center of China reported thyroid cancer with 466,100 new cases, ranking it as the most common cancer in third place [3]. Surgical treatment is the preferred choice due to its capability to remove thyroid lesions and lymph nodes [4]. Surgical treatment mainly includes traditional open surgery and endoscopic radical thyroidectomy [5678]. Traditional thyroid surgery is safe and effective but results in relatively large trauma and leaves surgical scars on the neck. With the improvement of cosmetic requirements, minimally invasive thyroid surgery aiming for smaller and hidden incisions is also followed. However, minimally invasive endoscopic thyroid surgery is a relatively new field for surgeons, and establishing the appropriate surgical space is challenging.
Venous thromboembolism (VTE) includes deep venous thrombosis (DVT) and pulmonary embolism (PE) [9]. DVT is defined as the formation of blood clots in the deep veins, most commonly occurring in the lower extremities [10]. Risk factors for DVT include obesity, clotting disorders, the presence of central venous lines, smoking, inactivity, congestive heart failure, major trauma, noninfectious inflammation, estrogen use, and pregnancy [1112]. Several clinical trials have also revealed an increased incidence of DVT in patients following surgery [1314]. Tian and Li [15] have found that the occurrence rate of DVT in patients undergoing gynecologic laparoscopic surgery is 11.55%. Furthermore, malignant tumors have been reported as significant risk factors for thrombosis in adults, with cancer patients having a relative risk of DVT 4.1–6.7 times higher than the general population [1617]. In a group of gastric or colorectal cancer patients in Japan, the incidence of DVT was 14% after a median observation period of 22 months [18]. DVT can adversely affect patients’ limb mobility, thereby impacting their ability to perform daily activities and reducing their quality of life [19]. Once a blood clot from DVT dislodges and enters the pulmonary circulation, it can cause PE and even death [202122]. Therefore, the prevention and treatment of DVT are currently a top research agenda.
For patients with thyroid cancer, the current clinical options include traditional thyroidectomy, as well as endoscopic minimally invasive thyroidectomy and lymph node dissection [23]. However, in clinical practice, we have found that both surgical treatment and postoperative chemotherapy can lead to the formation of DVT in the lower extremities, which can have varying degrees of impact on patients’ lives [24]. Therefore, this study involved 100 patients with thyroid cancer. Through the study of the efficacy of endoscopic and open surgery for thyroid cancer patients and the influence on their blood coagulation status, this study provided a basis for the selection of perioperative measures to prevent thrombosis.
The study was approved by the Ethics Committee of The First People’s Hospital of Yibin with a approval number of 2023 (30), and all patients signed consent forms. The methods were carried out in accordance with the approved guidelines.
One hundred patients with thyroid cancer who were admitted to the Department of General Surgery of our hospital from January 2021 to December 2021 were collected. Patients were randomly divided into an endoscopic group and an open surgery group by random number table method, with 50 cases in each group. The patients in the open surgery group were treated with traditional open surgery, and the patients in the endoscopic group were treated with endoscopic surgery.
The inclusion criteria were as follows: confirmation of pathological findings of papillary thyroid carcinoma; age less than 55 years old; body mass index <30 kg/m2; no neck surgery within the past 3 months; no history of pulmonary embolism or deep vein thrombosis; female patients who had not taken estrogen drugs within the past 6 months; absence of anticoagulant or procoagulant medication use; no bleeding or coagulation disorders; and all patients able to undergo surgery according to surgical indications.
The exclusion criteria included abnormal coagulation function, myocardial ejection fraction <55%, complications with diabetes, liver disease, or severe infection, incomplete clinical data, and patient refusal.
All the patients had complete preoperative preparation, and the anesthesia method was tracheal intubation and general anesthesia. The head was extended slightly back in the supine position, the shoulder pad was high, and the routine disinfection towel was laid.
The required endoscopic equipment was prepared, and the 3-hole method was adopted: small incisions of 0.5 cm were made along the bilateral areola, and a 0.5 cm trocar was inserted. A 1 cm incision was made to the right of the median of the front sternal bone, reaching to the deep fascia, and a 50 mL syringe was used to inject “dilatative fluid” into the incision (epinephrine:normal saline = 1:500), 100–200 mL was used to enter the subcutaneous layer of the incision with a special nondestructive stripper rod, and the subcutaneous tunnel and surgical space were established in a “fan-shaped” way toward the neck. After the “dilatant fluid” was extruded as much as possible, a trocar and 30° endoscope were implanted, and CO2 gas (pressure was about 6 mmHg) was injected. The cervical alba line and thyroid pseudocapsule were opened layer by layer by ultrasound knife to expose the whole thyroid gland, paying attention to protecting the recurrent laryngeal nerve and parathyroid gland, exposing the common carotid artery and internal jugular vein, and complete removal of the tumor. During cervical lymph node dissection, the head was turned to the healthy side, and a small incision was made about 1.0 cm above the clavicle on the affected side. Under the monitoring of the endoscopic display, after establishing a stable operating field of vision, the subcutaneous tissue was separated layer by layer by an ultrasonic knife. The submandibular gland was reached in the upper part of the neck, the lateral margin of the sternocleidomastoid muscle was reached on both sides, and the whole process of the internal jugular vein was exposed. The vagus nerve, accessory nerve, and common carotid artery were protected. Lymph nodes and adipose tissue in areas IIa, III, and IV were cleaned, and the operation field was rinsed to stop bleeding thoroughly. The drainage tube was drawn out of the body and the incision was sutured layer by layer with absorbable lines.
Conventional open surgery was performed, and a curved surgical incision of 4–10 cm at the 2 transverse fingers on the superior sternal notch was made. The subcutaneous fat was removed layer by layer, the platysma muscle was cut off, the false capsule was separated, and the tumor was removed completely after the glands were exposed. During the cervical lymph node dissection, the incision was extended along the affected side to the submandibular gland region, and the cervical lymph node dissection was performed. Postoperative hemostasis, drainage, and suture were performed [25].
Operation and postoperative indexes of the patients in the 2 groups were recorded in detail, including operation time, intraoperative blood loss, and length of hospital stay. Postoperative aesthetic satisfaction and complications of the patients in the 2 groups were also analyzed in detail, including postoperative incision infection, postoperative bleeding, superior laryngeal or recurrent laryngeal nerve injury, and parathyroid injury. Before surgery and 24 hours after surgery, 5 mL fasting venous blood was extracted from patients, centrifugation was performed for 10 minutes (1,500 revolutions/min, radius = 15 cm), and serum was separated and stored at −80 ℃ for use. The levels of aPTT, PT, and fibrinogen (FIB) were measured by the coagulation method and converted into international standard values according to the international normalized ratio (INR). Plasma D-dimer level was determined by enzyme-linked immunosorbent assay. All operations are carried out in strict accordance with the process.
Postoperative follow-up was conducted by telephone, return visit, and other methods. The follow-up included metastasis, recurrence, and thrombosis. The follow-up date was December 30, 2022.
After collecting and sorting out the relevant index data, statistical methods were used to analyze the results and research conclusions: IBM SPSS Statistics ver. 20.0 (IBM Corp.) was used for the statistical analysis of the data. The measurement data in this group were all subject to the normal distribution, expressed by mean ± standard deviation. Group t-test was used for comparison between groups, and paired t-test was used for comparison of indicators before and after surgery within the same group. The statistical data were expressed by number (%), and the comparison between the 2 groups was performed by the group chi-square test (test level, α = 0.05).
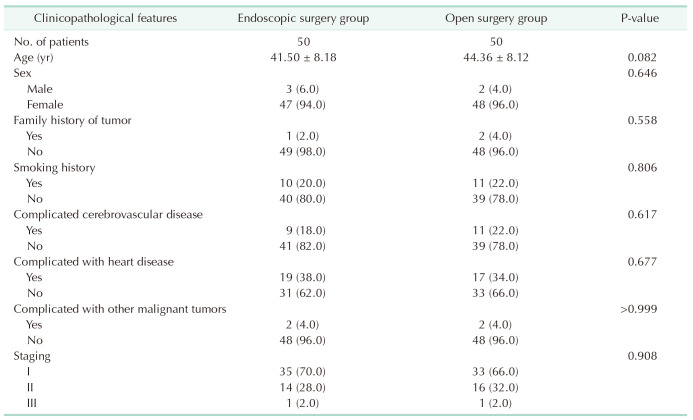
The average age of patients in the endoscopic group was 41.50 ± 8.18 years, with females accounting for 94% of the group. Twenty percent of the patients had a history of smoking, and 98% of the patients were classified as stage I or II. There were no statistically significant differences between the 2 groups in terms of age, sex, family history of tumors, smoking history, comorbidities of cerebrovascular diseases, cardiac diseases, other malignancies, or staging (P > 0.05, Table 1).
Compared to the open surgery group, the endoscopic group had significantly longer operation time (125.90 ± 9.78 minutes vs. 99.30 ± 7.22 minutes, P < 0.0001), while intraoperative blood loss (8.28 ± 3.48 mL vs. 47.50 ± 8.85 mL) and hospital stay (3.30 ± 1.09 days vs. 8.50 ± 2.17 days) were significantly reduced in the endoscopic group (P < 0.0001) (Table 2).
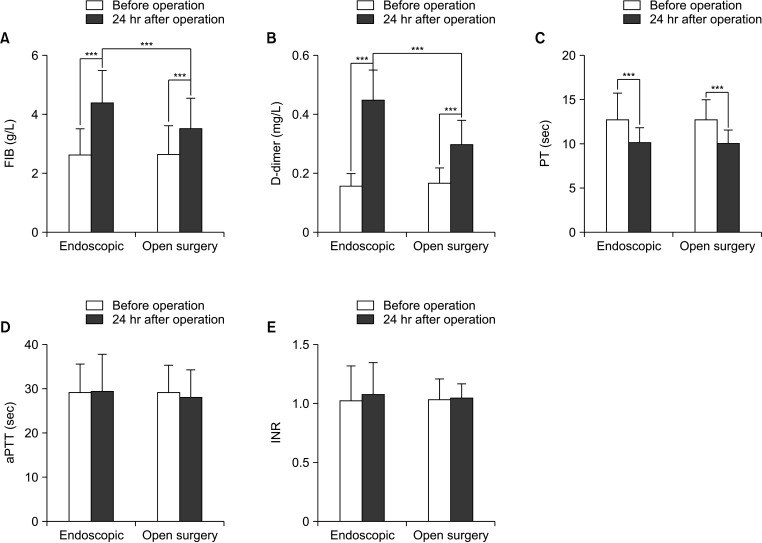
Compared to preoperative levels, FIB and D-dimer levels were significantly elevated in both the endoscopic and open surgery groups 24 hours after surgery (Fig. 1A, B). PT was significantly shortened (P < 0.0001) (Fig. 1C), while aPTT and INR showed no statistically significant differences (P > 0.05) (Fig. 1D, E). The comparison between the 2 groups at 24 hours after surgery showed that FIB and D-dimer levels were significantly higher in the endoscopic group than in the open surgery group (P < 0.0001), while PT, aPTT, and INR showed no statistically significant differences (P > 0.05) (Fig. 1).
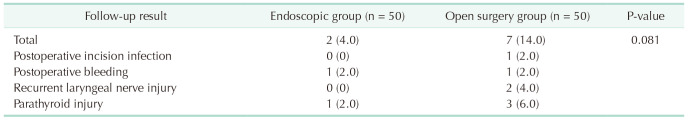
The average follow-up time for both groups was 18.61 ± 2.40 months. In the endoscopic group, there was 1 case of postoperative bleeding and 1 case of parathyroid injury. In the open surgery group, there was 1 case of postoperative wound infection, 1 case of postoperative bleeding, 2 cases of recurrent laryngeal nerve injury, and 3 cases of parathyroid injury. Although there were no statistically significant differences in complications between the 2 groups (P > 0.05), the endoscopic group had a lower incidence of complications (Table 3).
Out of 11 patients with metastasis, 5 were in the endoscopic group, all of which were lymph node metastasis. One patient underwent central lymph node dissection, and 2 patients received postoperative [131I] treatment. In the open surgery group, 6 patients had metastasis, with 4 cases of lymph node metastasis and 2 cases of lung metastasis. Three patients underwent central lymph node dissection, and 5 patients received postoperative [131I] treatment (Supplementary Table 1).
Eight patients experienced recurrence, which was confirmed through neck ultrasound and thyroglobulin antibody levels. Among them, 4 cases were in the endoscopic group, all of which did not undergo central lymph node dissection and did not receive postoperative [131I] treatment. In the open surgery group, 4 patients had recurrence, with 2 cases undergoing central lymph node dissection and 3 cases receiving postoperative [131I] treatment (Supplementary Table 2).
Six patients experienced thrombosis, all of which were DVTs. In the endoscopic group, 2 cases had thrombus in the intermuscular veins of the calf and did not undergo central lymph node dissection or receive postoperative [131I] treatment. In the open surgery group, there were 4 cases of thrombosis, including 2 cases in the intermuscular veins of the calf, 1 case in the peroneal veins, and 1 case in the posterior tibial veins. Two cases underwent central lymph node dissection, and 2 cases received postoperative [131I] treatment. The open surgery group had a higher incidence of thrombosis compared to the endoscopic group (Supplementary Table 3).
In the past decade, the incidence of thyroid cancer in the female population has been increasing by over 20% annually [26]. Thyroidectomy combined with lymph node dissection is an effective approach to inhibit the progression of the disease and prolong disease-free survival in patients. This study investigated perioperative indicators, fibrinolysis and coagulation markers before and after surgery, complications, and follow-up outcomes in the endoscopic group and the open surgery group. The results showed that the endoscopic group had significantly longer operation time compared to the open surgery group, while intraoperative blood loss and hospital stay were significantly lower in the endoscopic group. Both groups showed a significant increase in FIB and D-dimer levels and a significant reduction in PT at 24 hours after surgery compared to preoperative levels. FIB and D-dimer levels were significantly higher after surgery in the endoscopic group than in the open surgery group. Additionally, the endoscopic group had a lower incidence of complications compared to the open surgery group. Among the patients, 11 cases experienced metastasis, 8 cases experienced recurrence, and 6 cases experienced thrombosis.
He et al. [27] found that the surgical duration for the study group (endoscopic treatment) was longer than the control group (open surgery treatment). However, the amount of blood loss, 24-hour drainage through catheters, and length of hospital stay were significantly lower in the study group compared to the control group, which is consistent with our research findings. This study compared the effectiveness of endoscopic surgery and open surgery in treating thyroid cancer. The results revealed that patients in the endoscopic group had significantly lower intraoperative blood loss and shorter hospital stays than those in the open surgery group, which may be attributed to factors such as smaller incisions, less tissue dissection, and reduced tissue adhesion associated with endoscopic surgery [2829]. However, the surgical duration in the endoscopic group was longer than in the open surgery group, which we speculate is due to the increased difficulty of endoscopic procedures, inevitably resulting in longer operation times [30]. This necessitates surgeons to perform operations under laparoscopy to enhance proficiency, reduce surgical duration, and minimize risks. Additionally, the lower incidence of complications in the endoscopic group indicates that endoscopic surgery is safer and more reliable. This may be attributed to the high-definition display and larger field of view provided by endoscopic surgery, resulting in clearer visualization of thyroid anatomical structures and more precise thyroidectomy, thereby reducing the occurrence of inadvertent complications such as parathyroid missection, injury to major blood vessels, trachea, and nerves during the procedure [31].
Open surgery and endoscopic surgery are both invasive operations. Research has shown that this type of procedure can lead to postoperative DVT. However, there have been limited reports on the impact of these procedures on patients’ coagulation function [3032]. The coagulation process is a complex chain reaction involving a series of coagulation factors. D-dimer, FIB, PT, aPTT, and INR are common coagulation function evaluation indexes, which can reflect the level of coagulation factors and coagulation function by monitoring the coagulation indexes. D-dimer is the product of fibrin hydrolyzed by plasmin and can be used as an indicator to evaluate the fibrinolytic system of patients [333435]. The increase in D-dimer level indicates the active production and degradation of fibrin, and the body is in a hypercoagulable state [36]. In the final stage of coagulation, soluble FIB is transformed into insoluble FIB, thus causing blood coagulation, and FIB can reflect the state of coagulation function [3738]. PT is the time when prothrombin is converted into thrombin and plasma coagulates, which reflects the condition of the organism’s exogenous coagulation system [39]. In this study, PT and D-dimer levels of patients in the endoscopic and open surgery groups changed significantly before and after surgery, and the degree of change in the endoscopic group was higher than that in the open group. Compared to preoperative levels, both the endoscopic group and the open surgery group showed significant increases in FIB and D-dimer levels, as well as significant shortening of PT at 24 hours after the surgery. These findings suggest that both types of surgery can induce hypercoagulability and potentially lead to DVT. The hypercoagulable state in patients undergoing open surgery may be related to the anesthetic drugs or body position of surgical trauma, which could activate plasmin and thrombin in the body, thus affecting the blood status. After endoscopic surgery, patients exhibit a more pronounced hypercoagulable state, which may be attributed to the difficulties in lymph node dissection and prolonged surgical duration, both of which can stimulate vascular endothelial cells, activate the coagulation system, induce platelet activation and adhesion, resulting in a hypercoagulable state [40], this may also be associated with CO2 insufflation. In addition, the results of this study also showed that the FIB and D-dimer levels of the endoscopic group were higher than those of the open surgery group 24 hours after surgery, suggesting that the hypercoagulable state of blood was more obvious in endoscopic surgery, which may be related to the establishment of CO2 insufflation during the operation. The purpose of insufflation is to establish operable space for surgery and enlarge the operating field. However, CO2 insufflation can lead to complications such as hypercapnia and increased intracranial pressure [41]. In addition, during endoscopic surgery, carbon dioxide pressure can lead to significantly increased venous pressure, which restricts the return of venous blood, while slow venous blood flow and damage to vascular endothelial cells can activate the body’s coagulation system, leading to the imbalance between coagulation and anticoagulation, and contributing to the occurrence of hypercoagulation in patients [42].
Furthermore, studies have demonstrated that the internal jugular vein pressure and central venous pressure (CVP) increased significantly after aeration, and gradually decreased after aeration, indicating that CO2 pneumoperitoneum pressure in thoracoscopic thyroid surgery would indeed lead to a significant increase in internal jugular vein pressure and CVP [43]. The reason for the significant increase in internal jugular vein pressure may be that CO2 in the neck compresses the internal jugular vein, which leads to blocked blood flow back into most of the head and neck, resulting in increased pressure, blood stasis, and slow blood flow rate, thus increasing the possibility of thrombosis.
In our follow-up results, 11 patients experienced metastasis, and 8 patients experienced recurrence, which was unexpected. Possible reasons for this include incomplete surgical resection, lack of central compartment lymph node dissection, or absence of [131I] therapy, which may have resulted in incomplete removal of the lesions or incomplete inactivation of residual thyroid tissue, thereby increasing the risk of metastasis or recurrence. Additionally, 6 patients developed thrombosis, and we speculated that besides the impact of surgery on patients’ coagulation function, [131I] radiotherapy may also have an influence. There are reports suggesting that ionizing radiation affects the protein C pathway and its interaction with platelet regulatory proteins [44]. Following radiotherapy, procoagulant factors, activated factor VIII, proinflammatory nuclear factor κB, and D-dimer increase, and prothrombin fragments can promote procoagulant reactions [45]. Radiotherapy can also induce endothelial dysfunction and thrombus formation through the activation of Von Willebrand factor and the induction of primary hemostasis [46]. Moreover, in vitro studies have shown that tumor irradiation activates integrin αvβ3 in cancer cells [47], which plays a crucial role in cancer-related thrombosis.
Hence, it is necessary to take active measures to prevent the occurrence of postoperative DVT. Numerous studies have shown that the incidence of VTE can be reduced by 40% to 60% with the use of drug prophylaxis [4849]. At present, it is clinically recommended that patients should be treated with low molecular weight heparin, unsegmented heparin or pentaparin for preventive treatment according to the risk factors of VTE [50]. For patients with potential bleeding risk or severe bleeding, mechanical prophylaxis, gradient compression stockings, or intermittent inflatable compression may be given [51]. Research has also shown that postoperative patient immobility can lead to venous stasis and excessive expression of procoagulant factors in cancer, causing direct damage to the vascular endothelium [52]. Therefore, it is important to be aware of the risk of postoperative DVT in clinical practice. Measures can be taken to prevent this, such as reducing the duration of surgery and minimizing postoperative bed rest, particularly by promoting functional activity of the patient’s limbs. These measures aim to effectively prevent postoperative DVT and improve overall postoperative recovery.
This study has some limitations. The sample size of selected cases in this study is small, and this study is a single-center study. Therefore, further research with larger sample sizes and multi-center studies is needed to explore the risk factors of DVT in patients undergoing endoscopic thyroid surgery, so as to provide reliable evidence for the prevention of postoperative DVT. To sum up, while endoscopic surgery for thyroid cancer has benefits such as less intraoperative blood loss and faster recovery, its potential for hypercoagulability after endoscopic surgery is higher than traditional open surgery. Clinically, reasonable surgical methods should be selected based on the actual situation of patients, so as to give full play to the surgical effect and improve the quality of life of patients.
References
1. Kim J, Gosnell JE, Roman SA. Geographic influences in the global rise of thyroid cancer. Nat Rev Endocrinol. 2020; 16:17–29. PMID: 31616074.
2. Global Burden of Disease Cancer Collaboration. Fitzmaurice C, Dicker D, Pain A, Hamavid H, Moradi-Lakeh M, et al. The global burden of cancer 2013. JAMA Oncol. 2015; 1:505–527. PMID: 26181261.
3. Zheng RS, Chen R, Han BF, Wang SM, Li L, Sun KX, et al. Cancer incidence and mortality in China, 2022. Zhonghua Zhong Liu Za Zhi. 2024; 46:221–231. PMID: 38468501.
4. Qi L, Ping H, Cuisong L. Comparison on the clinical effects of endoscopy-assisted small neck incision surgery vs conventional thyroidectomy for the treatment of benign thyroid tumor. Clin Med Eng. 2020; 27:409–410.
5. Zhang X, Shi TP, Li HZ, Ma X, Wang BJ. Laparo-endoscopic single site anatomical retroperitoneoscopic adrenalectomy using conventional instruments: initial experience and short-term outcome. J Urol. 2011; 185:401–406. PMID: 21167534.
6. Cunchuan W, Cheng L. Progress of endoscopic thyroid surgery approach and indications. J Laparosc Surg. 2016; 21:241–245.
7. Wang Y, Xie QP, Yu X, Xiang C, Zhang ML, Zhao QZ, et al. Preliminary experience with transoral endoscopic thyroidectomy via vestibular approach: a report of 150 cases in a single center. Zhonghua Wai Ke Za Zhi. 2017; 55:587–591. PMID: 28789508.
8. Hou J, Guo BM, Jie K. Clinical comparison of the oral vestibular approach and complete areola approach for endoscopic thyroidectomy of unilateral thyroid carcinoma. J Laparosc Surg . 2019; 24:561–565.
9. Almarshad FM, Almegren M, Alshuaibi T, Alobaodi N, Almutawa A, Basunbl H, et al. Thromboprophylaxis after bariatric surgery. Blood Res. 2020; 55:44–48. PMID: 32269974.
10. Spiegl HR, Estepp JH, Li C, Gil S, Gosain A, Murphy AJ, et al. Risk for deep venous thrombosis in pediatric cancer patients undergoing surgery. J Pediatr Surg. 2021; 56:2360–2363. PMID: 33722369.
11. Tseng EK, Kolesar E, Handa P, Douketis JD, Anvari M, Tiboni M, et al. Weight-adjusted tinzaparin for the prevention of venous thromboembolism after bariatric surgery. J Thromb Haemost. 2018; 16:2008–2015. PMID: 30099852.
12. Gonzalez R, Haines K, Nelson LG, Gallagher SF, Murr MM. Predictive factors of thromboembolic events in patients undergoing Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2006; 2:30–36. PMID: 16925311.
13. Ahmad KS, Zayed ME, Faheem MH, Essa MS. Incidence of silent deep venous thrombosis after laparoscopic bariatric surgery in patients who received combined mechanical and chemical thromboprophylaxis compared to patients who received mechanical thromboprophylaxis only. Surg Innov. 2021; 28:144–150. PMID: 33035103.
14. Becattini C, Pace U, Pirozzi F, Donini A, Avruscio G, Rondelli F, et al. Rivaroxaban vs placebo for extended antithrombotic prophylaxis after laparoscopic surgery for colorectal cancer. Blood. 2022; 140:900–908. PMID: 35580191.
15. Tian Q, Li M. Risk factors of deep vein thrombosis of lower extremity in patients undergone gynecological laparoscopic surgery: what should we care. BMC Womens Health. 2021; 21:130. PMID: 33771148.
16. Khorana AA, Mackman N, Falanga A, Pabinger I, Noble S, Ageno W, et al. Cancer-associated venous thromboembolism. Nat Rev Dis Primers. 2022; 8:11. PMID: 35177631.
17. Heit JA, Silverstein MD, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ 3rd. Risk factors for deep vein thrombosis and pulmonary embolism: a population-based case-control study. Arch Intern Med. 2000; 160:809–815. PMID: 10737280.
18. Aonuma AO, Nakamura M, Sakamaki K, Murai T, Matsuda C, Itaya K, et al. Incidence of cancer-associated thromboembolism in Japanese gastric and colorectal cancer patients receiving chemotherapy: a single-institutional retrospective cohort analysis (Sapporo CAT study). BMJ Open. 2019; 9:e028563.
19. Moragón-Ledesma S, Galeano-Valle F, Calleja-Cartón E, Del-Toro-Cervera J, Demelo-Rodríguez P. Bilateral deep vein thrombosis, vena cava agenesis, and renal abnormalities: KILT syndrome: a case report and literature review. J Cardiovasc Transl Res. 2020; 13:629–631. PMID: 31773459.
20. Miccoli P, Berti P, Raffaelli M, Materazzi G, Baldacci S, Rossi G. Comparison between minimally invasive video-assisted thyroidectomy and conventional thyroidectomy: a prospective randomized study. Surgery. 2001; 130:1039–1043. PMID: 11742335.
21. Miccoli P, Minuto MN, Barellini L, Galleri D, Massi M, D’Agostino J, et al. Minimally invasive video-assisted thyroidectomy: techniques and results over 4 years of experience (1999-2002). Ann Ital Chir. 2004; 75:47–51. PMID: 15283387.
22. Hu S, Qi Y, Li B, Zhang G. Laparoscopic resection of benign thyroid masses: two cases. J Laparosc Surg. 2002; 7:91.
23. Qing L, Baoyan H, Xia L, Ruixian H. Evaluation of fast track surgery in perioperative nursing on neck dissection patients with papillary thyroid carcinoma. J Nurs Adm. 2020; 20:361–363.
24. Qiong W, Yu F, Zhuoyue L. Effect analysis of rapid rehabilitation nursing concept in perioperative nursing of thyroid cancer. Guizhou Med J. 2019; 43:1825–1826.
25. Chao D, Le Y, Chunsheng L, Lin L, Binlin M. Clinical effective comparison of radical operation of thyroid cancer under whole endoscope and traditional open surgery. J Xinjiang Med Univ. 2016; 39:338–341.
26. Zhang S, Sun K, Zheng R, Zeng H, Wang S, Chen R, et al. Cancer incidence and mortality in China, 2015. J Natl Cancer Cent. 2021; 1:2–11. PMID: 39036787.
27. He L, Qing F, Li M, Lan D. Effects of laparoscopic and traditional open surgery on the levels of IL-6, TNF-α, and Gal-3 in patients with thyroid cancer. Gland Surg. 2021; 10:1085–1092. PMID: 33842252.
28. Inabnet WB 3rd, Fernandez-Ranvier G, Suh H. Transoral endoscopic thyroidectomy-an emerging remote access technique for thyroid excision. JAMA Surg. 2018; 153:376–377. PMID: 29490360.
29. Lin P, Liang F, Cai Q, Han P, Chen R, Xiao Z, et al. Comparative study of gasless endoscopic selective lateral neck dissection via the anterior chest approach versus conventional open surgery for papillary thyroid carcinoma. Surg Endosc. 2021; 35:693–701. PMID: 32076863.
30. Becattini C, Pace U, Rondelli F, Delrio P, Ceccarelli G, Boncompagni M, et al. Rivaroxaban for extended antithrombotic prophylaxis after laparoscopic surgery for colorectal cancer. Design of the PRO-LAPS II STUDY. Eur J Intern Med. 2020; 72:53–59. PMID: 31818628.
31. Li Y, Zhou X. Comparison between endoscopic thyroidectomy and conventional open thyroidectomy for papillary thyroid microcarcinoma: a meta-analysis. J Cancer Res Ther. 2016; 12:550–555. PMID: 27461608.
32. Silva-Velazco J, Dietz DW, Stocchi L, Costedio M, Gorgun E, Kalady MF, et al. Considering value in rectal cancer surgery: an analysis of costs and outcomes based on the open, laparoscopic, and robotic approach for proctectomy. Ann Surg. 2017; 265:960–968. PMID: 27232247.
33. Shimizu K, Akira S, Jasmi AY, Kitamura Y, Kitagawa W, Akasu H, et al. Video-assisted neck surgery: endoscopic resection of thyroid tumors with a very minimal neck wound. J Am Coll Surg. 1999; 188:697–703. PMID: 10359365.
34. Gottlieb A, Sprung J, Zheng XM, Gagner M. Massive subcutaneous emphysema and severe hypercarbia in a patient during endoscopic transcervical parathyroidectomy using carbon dioxide insufflation. Anesth Analg. 1997; 84:1154–1156. PMID: 9141952.
35. Friedman JJ. The systemic circulation. Selkurt EE, editor. Physiology. 4th ed. Little Brown and Company;1976. p. 278–290.
36. Schöb OM, Allen DC, Benzel E, Curet MJ, Adams MS, Baldwin NG, et al. A comparison of the pathophysiologic effects of carbon dioxide, nitrous oxide, and helium pneumoperitoneum on intracranial pressure. Am J Surg. 1996; 172:248–253. PMID: 8862077.
37. Rubino F, Pamoukian VN, Zhu JF, Deutsch H, Inabnet WB, Gagner M. Endoscopic endocrine neck surgery with carbon dioxide insufflation: the effect on intracranial pressure in a large animal model. Surgery. 2000; 128:1035–1042. PMID: 11114640.
38. Silver I, Li B, Szalai J, Johnston M. Relationship between intracranial pressure and cervical lymphatic pressure and flow rates in sheep. Am J Physiol. 1999; 277:R1712–R1717. PMID: 10600918.
39. Inukai M, Usui Y. Clinical evaluation of gasless endoscopic thyroid surgery. Surg Today. 2005; 35:199–204. PMID: 15772789.
40. Braga M, Gianotti L, Vignali A, Di Carlo V. Immunonutrition in gastric cancer surgical patients. Nutrition. 1998; 14:831–835. PMID: 9834924.
41. Biao W, Guomin Z. Pathophysiological changes of endoscopic thyroid surgery. J Laparosc Surg. 2020; 15:575–577.
42. Rui T, Suyan G. Clinical analysis of deep vein thrombosis of lower extremity after gynecological laparoscopic surgery. Clin J Med Officers. 2012; 40:962–964.
43. Fengyun C, Yu G, Minzhong X, Nan L. The effects of carbon dioxide insufflation on internal jugular vein pressure and central venous pressure during endoscopic thyroidectomy via a breast approach. J Laparosc Surg. 2017; 22:201–203.
44. Geiger H, Pawar SA, Kerschen EJ, Nattamai KJ, Hernandez I, Liang HP, et al. Pharmacological targeting of the thrombomodulin-activated protein C pathway mitigates radiation toxicity. Nat Med. 2012; 18:1123–1129. PMID: 22729286.
45. Byrne M, Reynolds JV, O’Donnell JS, Keogan M, White B, Byrne M, et al. Long-term activation of the procoagulant response after neoadjuvant chemoradiation and major cancer surgery. Br J Cancer. 2010; 102:73–79. PMID: 19953092.
46. Boerma M, Kruse JJ, van Loenen M, Klein HR, Bart CI, Zurcher C, et al. Increased deposition of von Willebrand factor in the rat heart after local ionizing irradiation. Strahlenther Onkol. 2004; 180:109–116.
47. Leith JT, Hercbergs A, Kenney S, Mousa SA, Davis PJ. Activation of tumor cell integrin αvβ3 by radiation and reversal of activation by chemically modified tetraiodothyroacetic acid (tetrac). Endocr Res. 2018; 43:215–219. PMID: 29611723.
48. Stubbs MJ, Mouyis M, Thomas M. Deep vein thrombosis. BMJ. 2018; 360:k351. PMID: 29472180.
49. Boddi M, Peris A. Deep vein thrombosis in intensive care. Adv Exp Med Biol. 2017; 906:167–181. PMID: 27628009.
50. Duffett L, Kearon C, Rodger M, Carrier M. Treatment of superficial vein thrombosis: a systematic review and meta-analysis. Thromb Haemost. 2019; 119:479–489. PMID: 30716777.
51. Farge D, Frere C, Connors JM, Ay C, Khorana AA, Munoz A, et al. 2019 International clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer. Lancet Oncol. 2019; 20:e566–e581. PMID: 31492632.
52. Cool RM, Herrington JD, Wong L. Recurrent peripheral arterial thrombosis induced by cisplatin and etoposide. Pharmacotherapy. 2002; 22:1200–1204. PMID: 12222560.
SUPPLEMENTARY MATERIALS
Supplementary Tables 1–3 can be found via https://doi.org/10.4174/astr.2024.107.3.127.




 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download