Abstract
Acknowledgments
Notes
Data Availability Statement
All data analyzed during this study are included in this published article.
Authors’ Contribution
Conceptualization: SSN. Data curation: SSN, JM. Investigation: JM, EN, RAA. Methodology: SSN, JM. Project administration: SSN. Resources: JM. Software: AS. Supervision: SSN. Validation: SSN. Visualization: AS. Writing – original draft: JM, EN, SSN. Writing – review and editing: SSN, JM.
References
Fig. 1

Fig. 2
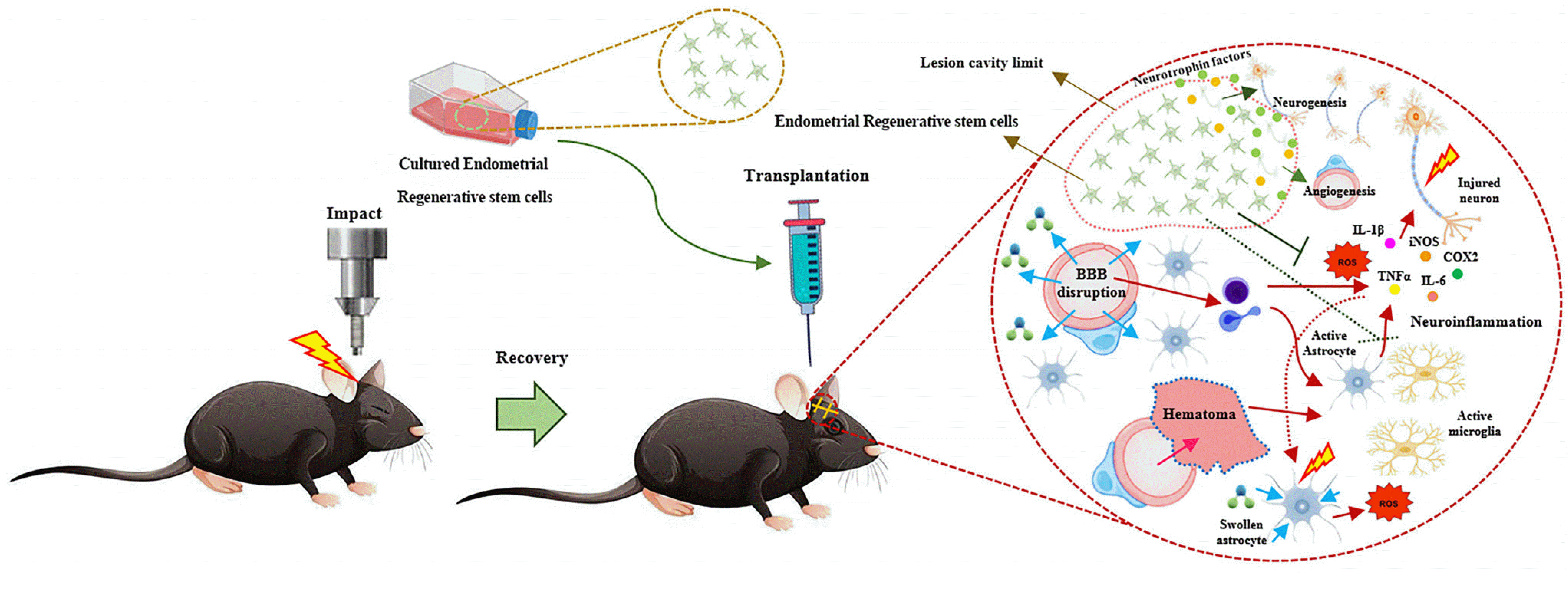
Table 1
| Sample | Type of study | Model | Cell source | Route and no. of injections | No. of cells | Result | Main finding | Reference | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | By curettage from reproductive-aged females undergoing surgery | In vitro | MPTP mouse model of PD | Adult hEnSCs | - | - | Exhibited neurogenic morphology, expression of the nestin and dopamine production TH and electrophysiological properties of neurons | hEnSCs differentiated into the dopamine-producing neurons | (35) |
| In vivo | Adult hEnSCs | Striatum 4 injections | 105 cells | Exhibit nestin, and human TH, survive in the location they are transplanted and spontaneously migrate to areas of damage and spontaneously differentiate in vivo | |||||
| 2 | Collected by hysterectomy and curettage from eight monkeys | In vivo | MPTP monkey model of Parkinson | Monkey EnSCs | Striatum 2 injections into right striatum |
4×106 cells |
Ability to migrate from the striatum and engraft in the substantia nigra. Also show significant migration to the contralateral side | EnSCs can migrate to the foci of cellular injury and differentiate to TH (+) neuron-like cells and protect endogenous dopaminergic neurons | (40) |
| 3 | Menstrual blood from healthy females donors | In vitro | On MPP+ treated cells | MenSCs-CM | - | - | Significantly reduce ROS generation and significantly reduce the number of cells in late apoptosis stage | MenSCs-CM can protect MPPC-induced cytotoxicity via reducing inflammation, oxidative stress, apoptosis and rescuing mitochondrial membrane potential | (41) |
| 4 | Collected with a Diva Cup from healthy females | In vivo | APP/PS1 transgenic mouse model of Alzheimer | hMenSCs | Bilaterally into the hippocampus | 105 cells | Reduced Ab deposition and decreased tau hyperphosphorylation | Decreased tau hyperphosphorylation | (36) |
| 5 | Menstrual blood sample | In vitro | OGD injury model of stroke | MenSCs | - | - | Cells were Nestin-positive, and readily differentiated into intermediate neuronal and astrocytic phenotype. Also, cells significantly reduced cell death and improved cell survival and elevated levels of trophic factors, such as VEGF, BDNF, and NT3 | The present neurostructural and behavioral benefits afforded by transplanted MenSCs | (37) |
| In vivo | Rat model of stroke | MenSCs | IC (3 injections, striatum), IV | 4×105 for IC, 4×106 for IV | significantly reduced behavioral abnormalities and increased survival of host cells | ||||
| 6 | Endometrial biopsy | In vivo | Mice model of autoimmune encephalomy-elitis | hEnSCs | Intraperitoneally | 1×106 | Suppress neuroinflammation | hEnSCs as a potent immunomodulatory tool for the treatment of autoimmune or neurodegenerative diseases | (24) |
| 7 | Uterine tubes and uterus were extracted from C57BL/6 WT and IDO−/− | In vitro | Co-culture of murine EnSCs and CD4+ T lymphocytes | Murine EnSCs | - | - | Reduced overall inflammation in the CNS, including mononuclear cells infiltrate, cytokine secretion and microglial activation | Suppressive activity of the unexplored murine EnSCs population, and shows the mechanism depends on IDO-Kynurenines-Aryl hydrocarbon receptor (AhR) axis | (42) |
| In vivo Murine model of autoimm-une encephal-omyelitis | Murine EnSCs | Intraperitoneally | 1×106 cells on days 0 and 10 post-immuniza-tion | ||||||
| 8 | Menstrual blood specimens were collected with menstrual cups | In vivo | Rat model of hemisected SCI | hMenSCs | Injection into the injured site | 1×105 | Improved the locomotor function, reduced the inflammatorycell infiltrations and vacuolization in the lesion site, increased neuronal markers in the lesion area, enhanced expression and secretion of BDNF, reduced scar formation, and decreased the expression of inflammatory cytokines | MenSCs transplantation promotion of the functional recovery of SCI rats via enhanced expression and secretion of BDNF, reduced scar formation and decreased the expression of inflammatory cytokine | (38) |
| 9 |
Human endometrial biopsy |
In vivo | Mouse model of hippocampal injury | EnSCs-derived extracellular vesicles | Intranasal | Total of 500 mg of EVs (released by 5×106 eMSCs) protein per kilogram of animal weight | Prevented histological damage and preserved speed locomotion and displacement changes presumably due to the growth factors contained in those vesicles | Intranasal administration of EnSCs – EVs could improve recovery of hippocampal tissue | (43) |
| 10 | Menstrual blood | In vivo | Mouse SCI model | hMenSCs | Intrathecal | 1×105 cell/μl and 2×105 cell | MenSCs transplantation Shh–induced MenSCs accelerated neuronal recovery, inhibited the formation ofglial cells and microglial activation atthe injured site, inhibited the expression of inflammatory factors, and improved the inflammatory microenvironment to achieve functional recovery of SCI | MenSCs transplantation improved functional recovery | (39) |
The main characteristics of experimental research in the application of endometrial regenerative cells (ERCs) in some neurological models.
PD: Parkinson’s disease, hEnSCs: human endometrial derived stem cells, TH: tyrosine hydroxylase, MenSCs: menstrual blood-derived stem cells, MenSCs-CM: conditioned medium of human menstrual blood derived endometrial stem cells, ROS: reactive oxygen species, OGD: oxygen glucose deprivation, VEGF: vascular endothelial growth factor, BDNF: brain-derived neurotrophic factor, NT3: neurotrophin-3, SCI: spinal cord injury, EVs: extracellular vesicles.
Table 2
| Cell type | Scaffold | Cell number | Markers | Duration of differentiation (day) | % Cell expression marker | Mechanism | Beneficial result | Main finding | Reference |
|---|---|---|---|---|---|---|---|---|---|
| hEnSCs | Tissue culture polystyrene (TCP) and poly ε-caprolactone (PCL) nanofibrous scaffold | 5×104 cells | ISL‐1, CHAT, islet-1, Pax6, NF-H, Hb9, and β‐tubulin III | 15 | 40∼90 | Significant upregulation of motor neuron markers | Exhibiting neural morphology and interconnection of cells | Improved motor neuron differentiation | (46) |
| hEnSCs | Biodegradable PLGA nanofiber scaffolds | 5×104 cells |
ISL‐1, CHAT, β-tubulin III NF-H HB9, pax6 |
15 | 40∼89 | Increased gene expression of markers | Exhibiting neural morphology and interconnection of cells | Improved motor neuron differentiation | (48) |
| hEnSCs | Electro spun Biocomposite Polycaprolactone/Collagen scaffolds | 5×104 cells | NF-H, TUJ-1, Chat, Islet-1, and HB9 | 15 | 60∼80 |
Inhibition of the PI3K/Akt pathway Neural marker expression |
Exhibiting neural morphology characteristics | Improved motor neuron differentiation | (47) |
| hEnSCs | Epothilone B (EpoB) | 2×105 cells |
ISL‐1, CHAT, and HB9 NES NEFH SYP, and β‐tubulin III proteins |
15 | More than 90 | Upregulation of motor neuron markers | Improved axonal length and neuronal alignment | Improved motor neuron differentiation | (49) |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download