Abstract
Purpose
Glycated hemoglobin (HbA1c) as a glycemic index may have limited value in pediatric patients with acute leukemia as they often present with anemia and/or pancytopenia. To address this issue, we evaluated the usefulness of glycated albumin (GA) as a glycemic monitoring index in pediatric patients with acute leukemia.
Methods
Medical records of 25 patients with type 2 diabetes mellitus (T2DM), 63 patients with acute leukemia, and 115 healthy children from Seoul St. Mary's Hospital, The Catholic University of Korea, were retrospectively investigated for serum GA, HbA1c, and fasting blood glucose (FBG) levels, along with demographic data.
Results
GA, HbA1c, and FBG levels did not differ between the control and acute leukemia groups. In the T2DM group, positive correlations were observed among GA, HbA1c, and FBG (P<0.01). Although GA level was not associated with the HbA1c level in the control group, GA and HbA1c levels showed a positive correlation in the acute leukemia group (P=0.045). Regression analysis revealed GA and HbA1c levels to be positively correlated in the acute leukemia and T2DM groups even after adjusting for age, sex, and body mass index z-score (P=0.007, P<0.01).
· Childhood leukemia survivors are at a higher risk for various short and long-term complications, including insulin resistance, obesity, and hyperglycemia.
· Glycated albumin (GA), an alternative marker of hyperglycemia, was positively correlated to glycated hemoglobin in pediatric patients with acute leukemia as in patients diagnosed with type 2 diabetes mellitus, suggesting that GA may serve as a complementary biomarker of glycemic status in this specific group.
Acute leukemia is the most common malignancy diagnosed during childhood. Advancements in treatment have led to a marked increase in survival rate, with some studies reporting survival rates of up to 90% depending on the risk group [1]. Issues related to the quality of life of survivors, including long-term complications such as hyperglycemia, insulin resistance, dyslipidemia, hypertension, obesity, and cardiovascular disease, have emerged as major concerns. Treatment methods for acute leukemia, which include drugs such as corticosteroids and asparaginase as well as cranial radiotherapy, can induce hyperglycemia and potentially cause childhood obesity during and/or following treatment [2]. Although the exact mechanism underlying these effects is unclear, these treatments may affect carbohydrate and lipid metabolism, resulting in insulin resistance and obesity [3]. Moreover, lack of physical activity and unhealthy eating habits may further increase the risks of these complications. Considering the adverse effects of these complications on the morbidity and mortality of pediatric patients, methods to minimize these sequelae are essential.
Hyperglycemia is often observed in patients undergoing chemotherapy. Transient hyperglycemia is especially prominent during remission induction therapy with glucocorticoid and asparaginase treatment and has been reported in up to 34% of pediatric patients with acute lymphoblastic leukemia (ALL) [4]. Steroid treatment may damage pancreatic beta cells; reduce insulin production; and increase insulin resistance, gluconeogenesis, and lipolysis, resulting in impaired glucose tolerance [5]. L-Asparaginase can also reduce insulin synthesis and secretion by depleting asparagine [6]. This drug is often associated with pancreatitis; it causes beta cell dysfunction and reduces insulin secretion [4]. Some of these effects are permanent and can lead to drug-induced diabetes [6]. Treatment of drug-induced diabetes includes careful glucose monitoring, diet modification, and insulin therapy when conservative management fails. Although most hyperglycemic events are transient, childhood leukemia survivors have a higher burden of long-term comorbidities, including diabetes mellitus [7]. Thus, close monitoring and follow up are crucial in this specific population.
Glycated hemoglobin (HbA1c) is a well-known marker of glycemic control that reflects the blood glucose status over the previous 2–3 months. Unfortunately, HbA1c level can be affected by the red blood cell (RBC) cycle [8]. As HbA1c is a product of the non-enzymatic chemical reaction of hemoglobin (Hb) with blood glucose, changes in Hb can affect HbA1c levels. Thus, hemolytic anemia, major blood loss, and blood transfusions may lead to misleading HbA1c results. RBC transfusion has been shown to reduce HbA1c levels [9]. Diseases such as anemia, hemoglobinopathies, and cystic fibrosis may also be associated with decreased HbA1c levels despite the presence of a high blood glucose level. Though debatable, a study found that HbA1c had low sensitivity for the detection of diabetes and impaired glucose tolerance in childhood cancer survivors treated with hematopoietic stem cell transplantation and total body irradiation [10]. Moreover, measurement methods of this marker varied widely among institutions [11]. To overcome these limitations, new markers of hyperglycemia have been investigated. Glycated albumin (GA) and fructosamine are products of non-enzymatic serum protein glycation and are independent of the RBC cycle. Glycation of albumin follows a relatively shorter cycle than that of Hb, and GA reflects the serum glucose level in the previous 2–3 weeks. These markers show faster improvement than HbA1c during recovery from acute diabetes ketoacidosis or diabetes onset [12].
However, there is a lack of data on the usefulness of GA in the pediatric population, particularly in patients with acute leukemia, who are at increased risk of diabetes mellitus compared to healthy peers [7]. Therefore, the objective of this study was to examine the relationship among GA, fasting blood glucose (FBG), and HbA1c levels in a pediatric population, particularly pediatric patients diagnosed with acute leukemia.
This retrospective study was conducted at Seoul St. Mary's Hospital, The Catholic University of Korea. Medical records of children aged 0–18 years who attended pediatric endocrinology outpatient and/or inpatient consultation sessions between January 2019 and May 2022 were included. A total of 203 children with GA measurement data was included in the analysis and divided into 3 groups: a normal control group, a type 2 diabetes mellitus (T2DM) group, and an acute leukemia group (Fig. 1).
The healthy control group consisted of children who visited the hospital for general health and growth examinations. A total of 115 children with available anthropometric data, as well as GA, FBG, and HbA1c levels, was included. Children with cardiovascular, neurological, and/or endocrine disorders and those on chronic systemic medications were excluded.
Children with documented GA, FBG, and HbA1c levels within 5 years from T2DM diagnosis were included in the T2DM group. Based on American Diabetes Association guidelines, T2DM was defined in children as either fasting plasma glucose level≥126 mg/dL, random plasma glucose level≥200 mg/dL with symptoms of polyuria or polydipsia, plasma glucose level≥200 mg/dL 2 hours after ingestion of glucose during an oral glucose tolerance test, or HbA1c≥6.5% in the absence of pancreatic cell antibodies [13]. Patients with T2DM with other systemic diseases, recent blood loss, or anemia were excluded from the study.
This study included 63 children diagnosed with primary acute leukemia who were referred to the pediatric endocrinology clinic for regular follow-up at least 6 months after completing treatment. Those with GA, FBG, and HbA1c measurements were included. Primary acute leukemia was classified as ALL or acute myeloid leukemia (AML) based on bone marrow or peripheral blood leukemic blast morphology and confirmed by immunophenotyping. All patients were treated with a uniform institutional ALL or AML protocol. Children with endocrine complications such as diabetes and thyroid dysfunction were excluded from the study. None of the patients had on-going medicines or treatments at the time of evaluation.
Laboratory data, including serum GA, HbA1c, and FBG levels; complete blood count; lipid profile; blood biochemistry; C-peptide level; and insulin level were obtained from same visit serum samples. Sex, age, height, weight, and body mass index (BMI) at the time of the visit were recorded. BMI was calculated as weight (kg) divided by height in meters squared (m2). Height, weight, and BMI were transformed into z-scores using the 2017 Korean National Growth Charts [14]. Obesity was defined as weight above the 95th percentile of the BMI SDS, which approximated to a z-score of 1.645. Furthermore, for children with T2DM and acute leukemia, medical chart reviews were conducted to assess age at diagnosis, treatment history, and hematopoietic stem cell transplantation status.
All statistical analyses were performed using IBM SPSS Statistics ver. 24.0 (IBM Corp., Armonk, NY, USA). Nonparametric values are expressed as median (interquartile range: 25%, 75%). Pearson/Spearman correlation analyses were performed to assess simple correlations. Multiple comparison analysis was corrected with post hoc Bonferroni correction. Linear regression was used for multivariable analysis. Level of significance was set at P<0.05. Post hoc power analysis was conducted to determine the statistical power of the study given the sample size and effect size. This study had a statistical power of 0.90.
Table 1 summarizes the basic characteristics of the study participants. A total of 203 pediatric subjects was included in the study: 63 with acute leukemia, 25 with T2DM, and 115 normal control participants. The median age of all participants was 11 years, and the median age of patients with T2DM was higher than that of the other 2 groups (P<0.001). Among the participants, 58.1% were male, 33.5% were obese, and the median BMI z-score was 0.69. BMI z-score was significantly higher in the T2DM group than in the other 2 groups (P<0.001). All 63 patients with acute leukemia had a history of packed RBC transfusion throughout treatment, with a median transfusion frequency of 12 prior to blood testing. In the T2DM group, 36% of the patients received a combination of insulin injections and oral medications. The median time from diagnosis to GA level sampling was 1.2 years (interquartile range, 0.09–2.15) for the T2DM group. The 115 normal control participants had either visited the clinic for growth assessment or for a general health checkup.
Table 2 presents the biochemical data of the study participants. Only GA and Hb levels differed significantly between the groups. GA level differed significantly between all 3 groups, with the highest level found in the T2DM group (P<0.001). HbA1c, FBG, and total protein levels and the homeostasis model of insulin resistance (HOMA-IR) score were higher in the T2DM group than in the other 2 groups, but the values did not differ between the acute leukemia and normal control groups. White blood cell, Hb, and platelet levels were lower in the acute leukemia group than the other 2 groups (P=0.003, P<0.001, P<0.001, respectively). C-peptide levels did not differ significantly among the groups.
GA and HbA1c levels showed positive correlations (r=0.926, P<0.01) in the T2DM group. Moreover, FBG level was positively correlated with GA (r=0.821, P<0.001) and HbA1c (r=0.773, P<0.001) levels. While HbA1c and FBG levels were positively correlated in the normal control group as expected (r=0.275, P=0.003), GA level was not associated with the HbA1c level in the normal control group (r=-0.02, P=0.824). In the acute leukemia group, however, HbA1c level and FBG as well as GA and HbA1c levels were positively correlated (r=0.264, P=0.037 and r=0.253, P=0.045, respectively) (Fig. 2). Further subgroup analysis of the normoglycemia group (HbA1c<5.7%) and prediabetes group (5.7%≤HbA1c≤6.4%) within the acute leukemia group revealed a positive correlation between HbA1c and GA in the prediabetes group (P=0.005), while no correlation was found in the normal control group (P=0.962) (Supplementary Fig. 1).
GA level was negatively associated with BMI z-score in the control group (r=0.554, P<0.001). However, GA level showed no correlation with BMI z-score in the acute leukemia and T2DM groups (r=-0.152, P=0.236 and r=-0.292, P=0.156, respectively). HbA1c level was positively related to BMI z-score in the control group (r=0.24, P=0.01), but the 2 parameters showed no association in the other 2 groups (Fig. 3).
Additional correlation analysis of HbA1c and Hb levels showed no significant correlations in any of the 3 groups (T2DM: r=0.143, P=0.515; control: r=-0.134, P=0.155; acute leukemia: r=0.100, P=0.438). Similarly, no significant correlations were found between GA and albumin levels in the 3 groups (T2DM: r=-0.109, P=0.620; control: r=-0.067, P=0.480; acute leukemia: r=-0.209, P=0.201). Moreover, there were no correlations between transfusion frequency and GA or HbA1c levels in patients with acute leukemia (P=0.859 and P=0.547, respectively).
A linear regression model was used to determine the association between HbA1c and GA levels in each group (Table 3). After adjusting for age, sex, and BMI z-score, GA level showed a positive correlation with HbA1c level in both the acute leukemia and T2DM groups (P=0.007 and P<0.01, respectively). Furthermore, when adjusted for multiple covariates, FBG level was positively correlated with HbA1c level in the T2DM (P<0.001) and acute leukemia groups (P<0.001). In the normal group, FBG level was not significantly correlated with HbA1c level (P=0.065).
To the best of our knowledge, this is the first study to investigate the potential relationship between GA and HbA1c levels in pediatric patients with acute leukemia. In our study, GA, FBG, and HbA1c levels showed strong correlations with T2DM in pediatric patients, as expected. Although the GA level was only slightly lower in the acute leukemia group, it was positively correlated with the HbA1c level in the acute leukemia group and the T2DM group but not in the normal control group. Notably, GA was positively associated with increases in HbA1c levels in patients with acute leukemia as in patients with T2DM, even after adjustment for age, sex, and BMI z-score. Given the elevated risk of T2DM in children treated for acute leukemia, vigilant surveillance for endocrine complications is important. Our findings suggest that GA measurement may have a role in patients with acute leukemia and can complement conventional methods for evaluating glycemic status in this specific population.
GA level has been shown to be highly correlated with HbA1c level in previous studies of patients with diabetes. In adult studies, GA was a highly specific but less sensitive marker of hyperglycemia than HbA1c or FBG levels [15]. However, several previous studies have noted that HbA1c is a poor marker for glucose monitoring in gestational diabetes mellitus (GDM) as pregnancy occurs over the relatively short time period of 40 weeks and can involve iron deficiency [16]. Sugawara et al. suggested that measurement of the GA level and the GA/HbA1c ratio were more useful than the HbA1c level for predicting infant complications due to GDM [17]. The demand for new biomarkers has led to several studies of GA. Kim et al. [18] reported that a GA level > 16% was associated with poorer outcomes in stroke patients without diabetes and was a prognostic biomarker for short-term stroke outcomes. Another study recommended GA as a possible alternative biomarker of glycemic status in patients with end-stage renal disease who have altered glucose and insulin homeostasis due to decreased renal metabolism [19]. As GA reflects recent glycemic status and can identify glycemic changes before fluctuations in the HbA1c level, it may better assess glucose variability [20]. A prospective study by Desouza et al. [21] found GA to be a better short-term correlation factor for blood glucose changes than other glycemic indices. However, while studies in pediatric patients with diabetes also showed that GA better reflected the glycemic status than HbA1c as in adult studies, studies of GA in the general pediatric population are limited and controversial [11].
We found that GA and HbA1c levels were correlated in both the acute leukemia and T2DM groups. While the correlation between GA and HbA1c is well documented in adult populations, findings in pediatric populations are scarce and often contradictory. A Japanese study of a pediatric population without diabetes reported a surprising inverse correlation between GA and HbA1c (r=-0.139, P<0.001) [22]. More recently, a large study from the US found GA to be poorly correlated with traditional markers of hyperglycemia in healthy adolescents with normoglycemia or mild hyperglycemia [23]. In our study, while GA and HbA1c did not demonstrate any significant correlation in the normal control group, GA was significantly correlated with HbA1c in the acute leukemia group. Subgroup analysis also revealed significant correlation of HbA1c and GA in the prediabetes subgroup of the acute leukemia group but not in the prediabetes subgroup of the normal control group (P=0.005). Prior studies in adults have shown that GA measurements are weakly correlated with HbA1c and FBG measurements at lower levels but showed a strong correlation at higher levels [24]. This may explain the lack of correlation seen in the healthy pediatric group. Nevertheless, GA was significantly correlated with HbA1c in the acute leukemia group as in the T2DM group. Neither the normal control group nor acute leukemia group had been diagnosed with diabetes mellitus or any glucose metabolism problem. Previous studies have demonstrated that insulin resistance is affected by prolonged corticosteroid use [2]. Other chemotherapeutic drugs, such as L-asparaginase, can also alter glucose metabolism and lead to a spectrum of hyperglycemia, ranging from transient episodes to diabetic ketoacidosis or prolonged diabetes, which can impact survival outcomes [25]. The combination effect of various treatment-related chemotherapeutic agents and proinflammatory cytokines is known to contribute to various metabolic disturbances in these specific groups [26]. One study found no difference in obesity status among pediatric patients with leukemia with or without insulin resistance (obese, 45.2%; normal weight, 41.9%) [27]. This suggests that insulin resistance may occur in children with leukemia regardless of their obesity status. Such hyperglycemic events and insulin resistance may arise in children with acute leukemia as a result of the long-term synergistic effects of various medications. Suwa et al. [28] showed that GA levels and continuous glucose monitoring parameters were significantly correlated, suggesting the need for wider application of GA as a glucose monitoring marker, particularly in susceptible populations. Although most hyperglycemia events reported in acute leukemia treatment are usually transient, they may be associated with adverse long-term health outcomes [29]. Studies have found that not only do childhood cancer survivors suffer from a higher incidence of diabetes mellitus compared to the general population, but also that the age-specific cumulative prevalence of metabolic syndrome increases rapidly with age in this specific group [30,31]. Given that traditional glycemic markers may not adequately reflect glucose variability in specific circumstances, there is a pressing need for alternative glucose monitoring markers. While our study found that the GA level was not significantly correlated with FBG level, the samples may not have been from fasting serum and may not have reflected the average glucose levels of the subjects, as mentioned above. Furthermore, previous studies have shown that both HbA1c and GA correlate well with average glucose level on continuous glucose monitoring (CGM) rather than single-point glucose measurements [32]. Desouza et al. [33] reported GA to be a more sensitive marker of daily glycemic excursions than HbA1c in patients with diabetes. Unfortunately, due to the retrospective design of our study, we were unable to obtain data on average glucose levels or measure glucose variability via CGM. As FBG only represents a single-point serum glucose concentration, it may not reflect average glucose variability, which could explain the lack of correlation in the acute leukemia group. Nevertheless, we found a significant correlation between GA and HbA1c levels in both acute leukemia and T2DM groups, suggesting GA as a potential complementary glycemic marker in situations where short-term glucose monitoring and careful screening for hyperglycemia are required. Further studies with larger sample sizes and more diverse populations, such as pediatric patients with acute leukemia diagnosed with diabetes mellitus, are necessary to fully understand the role of GA in glucose monitoring and the discrepancies that may exist among patient groups.
At the beginning of the study, we hypothesized that a history of multiple transfusions in pediatric patients with acute leukemia may have affected their HbA1c level. Sugimoto et al. found that autologous blood donation can reduce the HbA1c level in patients with T2DM [34]. The authors speculated that RBC turnover was accelerated following blood donation and caused a reduction in the HbA1c level [18]. Thus, HbA1c may not be a satisfactory indicator of actual glycemic control in cases with a history of blood transfusions. However, although levels of Hb were significantly lower in the acute leukemia group than in the other 2 groups, Hb and HbA1c did not show a significant correlation (P=0.44), in contrast to previous studies where the authors found that HbA1c was inversely correlated with iron deficiency anemia [35]. While all 63 patients with acute leukemia had a history of RBC transfusion, they were not undergoing "active" chemotherapy at the time of their visit to our clinic. Rather, patients had completed chemotherapy and/or transplantation at least several months to years prior. Nevertheless, the previous transfusion history as well as complex treatment history of these patients may result in life-long sequelae in these patients. The extent to which HbA1c and GA influence glucose metabolism in patients with acute leukemia remains unclear, as does the impact of their previous transfusion and treatment histories. GA may serve as an alternative glycemic measure in situations where HbA1c measurements are unreliable in children treated for cancer. Conducting further research involving patients on active chemotherapy, who frequently present with anemia and pancytopenia, may enable a better understanding of glucose metabolism in this population.
In a nationwide study conducted in the US, GA level was inversely associated with BMI and cardiometabolic risk factors [23]. A previous study in a non-diabetic adult population also reported that BMI was negatively correlated with GA [36]. Other studies have reported controversial results regarding the association between obesity and GA. In our study, BMI z-score showed an inverse relationship with GA level in normal children. Obesity and albumin turnover may be associated, and chronic low-grade systemic inflammation may increase the catabolic albumin turnover rate in obese patients [24,38,39]. Kim et al. [18] suggested that the association between GA level and low adiposity meant that GA was a less reliable marker at normal levels of GA. Increased inflammation in obese children may provide another possible explanation for the negative correlation between BMI z-score and GA. Koga et al. [36] suggested that plasma high-sensitivity C-reactive protein (hs-CRP), an obesity-related inflammatory marker, could play a role. The authors hypothesized that obesity-related cytokines promote hs-CRP synthesis in the liver, resulting in higher hs-CRP levels in obese patients compared to the non-obese group, The authors showed that BMI as well as hs-CRP were negatively associated with GA in non-diabetic patients. However, we did not observe a relationship between GA level and BMI status in pediatric patients with acute leukemia nor in patients with diabetes. Although we were unable to assess hs-CRP levels in our study, other non-glycemic factors may explain the association between GA and BMI z-scores, and further studies are warranted.
This study had several limitations. First, it relied on data from retrospective chart review, meaning that some participants had missing data, and there may have been differences in sampling times due to the reliance on participants to follow the instructions to have samples drawn early in the morning. Some patients' caregivers may not have followed the instructions and the patient may not have fasted. Second, we did not evaluate other parameters associated with obesity and metabolic syndrome. A more detailed evaluation of adipose tissue and additional parameters, including free fatty acids, hs-CRP, and nutritional status, may be required to analyze the relationships between GA and other factors. Third, the sample size was small and varied greatly among groups. Despite having a statistical power of 0.9, our acute leukemia group comprised only a small percentage of leukemia patients who visited the endocrinology center and underwent GA, FBG, and HbA1c evaluation from a single blood sample. Furthermore, due to the limitation of a retrospective design, we were unable to obtain a uniform sample size across groups. To further examine the relationship between GA and HbA1c, a prospective larger population group study is necessary. Another limitation of our study was that obese children may have been overrepresented in the normal control group. Recent studies in Korea have found a high prevalence obesity in children (between 17.2% and 31.4%, depending on the study) [39,40]. The percentage of obesity in the normal control group in our study was 32%. This may be because the study was conducted at the pediatric endocrinology department, where parents who are concerned with their children's weight visit compared to the actual obesity prevalence. Finally, all patients in the acute leukemia group were stable, having completed treatment months to years prior to when serum measurements and anthropometric data were obtained. Further studies of GA levels during the active treatment phase and long-term monitoring would contribute to a better understanding of glucose metabolism in this specific cohort. A strength of our study is that it is the first to demonstrate a positive relationship between GA and HbA1c levels in the pediatric acute leukemia population.
In conclusion, our findings suggests that GA can serve as a complementary biomarker of glycemic status in pediatric patients with acute leukemia, as well as in patients with diabetes. However, given the limitations of our study, further research is needed to determine the usefulness of GA as a reliable glycemic index in pediatric patients with acute leukemia. Such investigations could shed more light on the potential clinical applications of GA in the management of glycemic control in these patients.
Supplementary Material
Supplementary Fig. 1 can be found via https://doi.org/10.6065/apem.2346100.050.
Supplementary Fig. 1.
Subgroup correlation between the fasting blood glucose level and the HbA1c and glycated albumin levels in control (A) and acute leukemia (B). Normoglycemia (HbAlc < 5.7%), prediabetes (5.7% ≤ HbA1c ≤ 6.4%). HbA1c, glycated hemoglobin.
Notes
Funding
This study was funded by Multicenter Networks for Ideal Out-comes of Pediatric Rare Endocrine and Metabolic Disease in Korea (OUTSPREAD Study), research number 5-2022-D0737-00007.
References
1. Kononczuk K, Muszynska-Roslan K, Konstantynowicz-Nowicka K, Krawczuk-Rybak M, Chabowski A, Latoch E. Biomarkers of glucose metabolism alterations and the onset of metabolic syndrome in survivors of childhood acute lymphoblastic leukemia. Int J Mol Sci. 2022; 23:3712.

2. Bahoush G, Salajegheh P, Rohani F. Association between body mass index and insulin resistance in survivors of pediatric acute lymphoblastic leukemia. Leuk Res Rep. 2020; 13:100199.

3. Pluimakers VG, van Waas M, Neggers S, van den Heuvel-Eibrink MM. Metabolic syndrome as cardiovascular risk factor in childhood cancer survivors. Crit Rev Oncol Hematol. 2019; 133:129–41.

4. Sonabend RY, McKay SV, Okcu MF, Yan J, Haymond MW, Margolin JF. Hyperglycemia during induction therapy is associated with poorer survival in children with acute lymphocytic leukemia. J Pediatr. 2009; 155:73–8.

5. Gregoriou K, Craigie I, Gibson B, Mason A, Shaikh MG. Risk factors and management of corticosteroid-induced hyperglycaemia in paediatric acute lymphoblastic leukaemia. Pediatr Blood Cancer. 2020; 67:e28085.
6. Handattu K, Sharma LK, Vijayasekharan K, Bhat KV, Aroor S, Sudhanshu S. Drug induced diabetes mellitus in pediatric acute lymphoblastic leukemia: approach to diagnosis and management. J Pediatr Hematol Oncol. 2022; 44:273–9.

7. Williams HE, Howell CR, Chemaitilly W, Wilson CL, Karol SE, Nolan VG, et al. Diabetes mellitus among adult survivors of childhood acute lymphoblastic leukemia: a report from the St. Jude Lifetime Cohort Study. Cancer. 2020; 126:870–8.

8. Chan CL, Pyle L, Kelsey M, Newnes L, Zeitler PS, Nadeau KJ. Screening for type 2 diabetes and prediabetes in obese youth: evaluating alternate markers of glycemia - 1,5-anhydroglucitol, fructosamine, and glycated albumin. Pediatr Diabetes. 2016; 17:206–11.

9. Spencer DH, Grossman BJ, Scott MG. Red cell transfusion decreases hemoglobin A1c in patients with diabetes. Clin Chem. 2011; 57:344–6.

10. Wei C, Unsworth R, Davis N, Cox R, Bradley K, Stevens M, et al. Survivors of childhood leukaemia treated with haematopoietic stem cell transplantation and total body irradiation should undergo screening for diabetes by oral glucose tolerance tests. Diabet Med. 2016; 33:1347–51.

11. Chan CL, McFann K, Newnes L, Nadeau KJ, Zeitler PS, Kelsey M. Hemoglobin A1c assay variations and implications for diabetes screening in obese youth. Pediatr Diabetes. 2014; 15:557–63.

12. Musha I, Mochizuki M, Kikuchi T, Akatsuka J, Ohtake A, Kobayashi K, et al. Estimation of glycaemic control in the past month using ratio of glycated albumin to HbA1c. Diabet Med. 2018; 35:855–61.
13. American Diabetes A. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2019. Diabetes Care. 2019; 42(Suppl 1):S13–28.
14. Kim JH, Yun S, Hwang SS, Shim JO, Chae HW, Lee YJ, et al. The 2017 Korean National Growth Charts for children and adolescents: development, improvement, and prospects. Korean J Pediatr. 2018; 61:135–49.

15. Zemlin AE, Barkhuizen M, Kengne AP, Erasmus RT, Matsha TE. Performance of glycated albumin for type 2 diabetes and prediabetes diagnosis in a South African population. Clin Chim Acta. 2019; 488:122–8.

16. Hashimoto K, Osugi T, Noguchi S, Morimoto Y, Wasada K, Imai S, et al. A1C but not serum glycated albumin is elevated because of iron deficiency in late pregnancy in diabetic women. Diabetes Care. 2010; 33:509–11.

17. Sugawara D, Sato H, Makita E, Kuwata T, Takagi K, Ichihashi K. Clinical usefulness of glycated albumin and glycated albumin-to-glycated hemoglobin ratio of gestational diabetes mellitus in late pregnancy for predicting infant complications. Pediatr Neonatol. 2022; 63:239–46.

18. Kim Y, Lee SH, Kang MK, Kim TJ, Jeong HY, Lee EJ, et al. Glycated albumin, a novel biomarker for short-term functional outcomes in acute ischemic stroke. Brain Sci. 2021; 11:337.

19. Vos FE, Schollum JB, Walker RJ. Glycated albumin is the preferred marker for assessing glycaemic control in advanced chronic kidney disease. NDT Plus. 2011; 4:368–75.

20. Rescalli A, Varoni EM, Cellesi F, Cerveri P. Analytical challenges in diabetes management: towards glycated albumin point-of-care detection. Biosensors (Basel). 2022; 12:687.
21. Desouza CV, Holcomb RG, Rosenstock J, Frias JP, Hsia SH, Klein EJ, et al. Results of a study comparing glycated albumin to other glycemic indices. J Clin Endocrinol Metab. 2020; 105:677–87.

22. Nishimura R, Kanda A, Sano H, Matsudaira T, Miyashita Y, Morimoto A, et al. Glycated albumin is low in obese, non-diabetic children. Diabetes Res Clin Pract. 2006; 71:334–8.

23. Wallace AS, Rooney MR, Brady TM, Echouffo-Tcheugui JB, Christenson R, Grams ME, et al. The performance of glycated albumin as a biomarker of hyperglycemia and cardiometabolic risk in children and adolescents in the United States. Pediatr Diabetes. 2022; 23:237–47.

24. Ribeiro RT, Macedo MP, Raposo JF. HbA1c, Fructosamine, and glycated albumin in the detection of dysglycaemic conditions. Curr Diabetes Rev. 2016; 12:14–9.

25. Pollock NI, Flamand Y, Zhu J, Millington K, Stevenson K, Silverman LB, et al. Hyperglycemia during induction therapy for acute lymphoblastic leukemia is temporally linked to pegaspargase administration. Pediatr Blood Cancer. 2022; 69:e29505.

26. Chueh HW, Yoo JH. Metabolic syndrome induced by anticancer treatment in childhood cancer survivors. Ann Pediatr Endocrinol Metab. 2017; 22:82–9.

27. Kartal I, Alacam A, Dagdemir A, Kara C, Dincer OS, Albayrak C, et al. Frequency of obesity and metabolic syndrome in childhood leukemia and lymphoma survivors. Diabetol Metab Syndr. 2022; 14:16.

28. Suwa T, Ohta A, Matsui T, Koganei R, Kato H, Kawata T, et al. Relationship between clinical markers of glycemia and glucose excursion evaluated by continuous glucose monitoring (CGM). Endocr J. 2010; 57:135–40.

29. McCormick MC, Sharp E, Kalpatthi R, Zullo J, Gurtunca N, Zhang J, et al. Hyperglycemia requiring insulin during acute lymphoblastic leukemia induction chemotherapy is associated with increased adverse outcomes and healthcare costs. Pediatr Blood Cancer. 2020; 67:e28475.

30. Saultier P, Auquier P, Bertrand Y, Vercasson C, Oudin C, Contet A, et al. Metabolic syndrome in long-term survivors of childhood acute leukemia treated without hematopoietic stem cell transplantation: an L.E.A. study. Haematologica. 2016; 101:1603–10.

31. Brignardello E, Felicetti F, Castiglione A, Chiabotto P, Corrias A, Fagioli F, et al. Endocrine health conditions in adult survivors of childhood cancer: the need for specialized adult-focused follow-up clinics. Eur J Endocrinol. 2013; 168:465–72.

32. Piona C, Marigliano M, Mozzillo E, Rosanio F, Zanfardino A, Iafusco D, et al. Relationships between HbA1c and continuous glucose monitoring metrics of glycaemic control and glucose variability in a large cohort of children and adolescents with type 1 diabetes. Diabetes Res Clin Pract. 2021; 177:108933.

33. Desouza CV, Rosenstock J, Zhou R, Holcomb RG, Fonseca VA. Glycated albumin at 4 weeks correlates with a1c levels at 12 weeks and reflects short-term glucose fluctuations. Endocr Pract. 2015; 21:1195–203.

34. Sugimoto T, Hashimoto M, Hayakawa I, Tokuno O, Ogino T, Okuno M, et al. Alterations in HbA1c resulting from the donation of autologous blood for elective surgery in patients with diabetes mellitus. Blood Transfus. 2014; 12 Suppl 1(Suppl 1):s209–13.
35. Janice D, Prathima MB, Sushith S, Narayanan R, Reshma S, Nair S, et al. Effect of iron deficiency anaemia over glycated hemoglobin in non-diabetic women. Int J Biochem Mol Biol. 2022; 13:23–7.
36. Koga M, Otsuki M, Matsumoto S, Saito H, Mukai M, Kasayama S. Negative association of obesity and its related chronic inflammation with serum glycated albumin but not glycated hemoglobin levels. Clin Chim Acta. 2007; 378:48–52.

37. Kim M, Kim J. Cardiometabolic risk factors and metabolic syndrome based on severity of obesity in Korean children and adolescents: data from the Korea National Health and Nutrition Examination Survey 2007-2018. Ann Pediatr Endocrinol Metab. 2022; 27:289–99.

38. Kim JH, Lim JS. The association between C-reactive protein, metabolic syndrome, and prediabetes in Korean children and adolescents. Ann Pediatr Endocrinol Metab. 2022; 27:273–80.

39. Park HK, Seo JY, Jung HW, Lim JS. Prevalence and trends in obesity and severe obesity in Korean children and adolescents, 2007-2020: a population-based study. Pediatr Int. 2023; 65:e15472.

40. Kang HM, Jeong DC, Suh BK, Ahn MB. The impact of the coronavirus disease-2019 pandemic on childhood obesity and vitamin D status. J Korean Med Sci. 2021; 36:e21.
Fig. 1.
Flowchart of study population. MODY, monogenic diabetes; TIDM, type 1 diabetes melliuts; T2DM, type 2 diabetes melliuts.
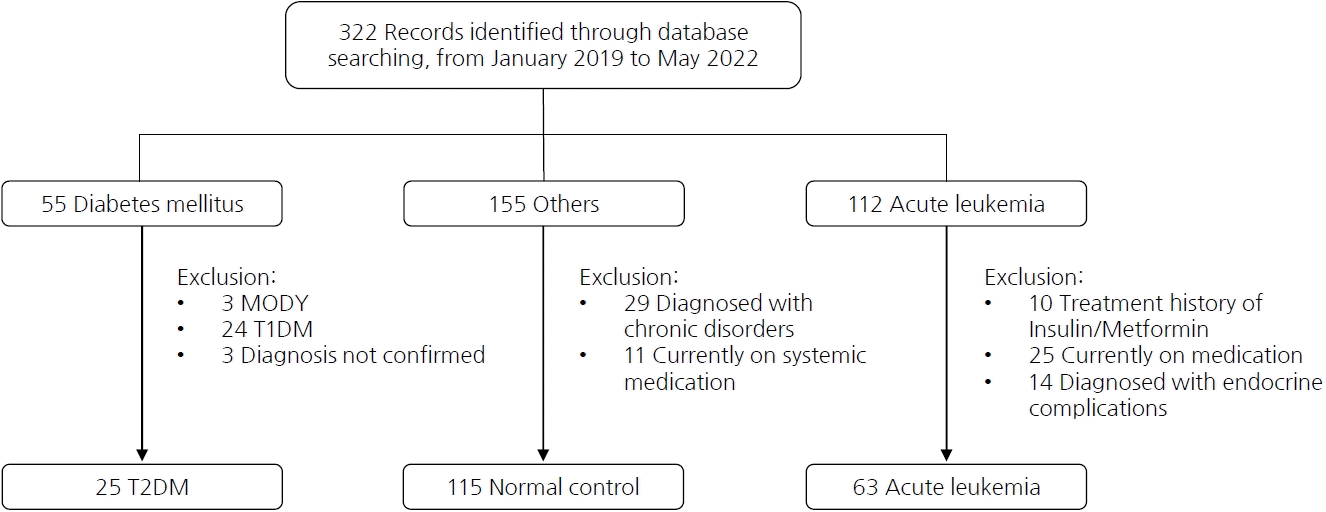
Fig. 2.
Correlation between the fasting blood glucose level and the HbA1c and glycated albumin levels in each study group. HbA1c, glycated hemoglobin; T2DM, type 2 diabetes melliuts.
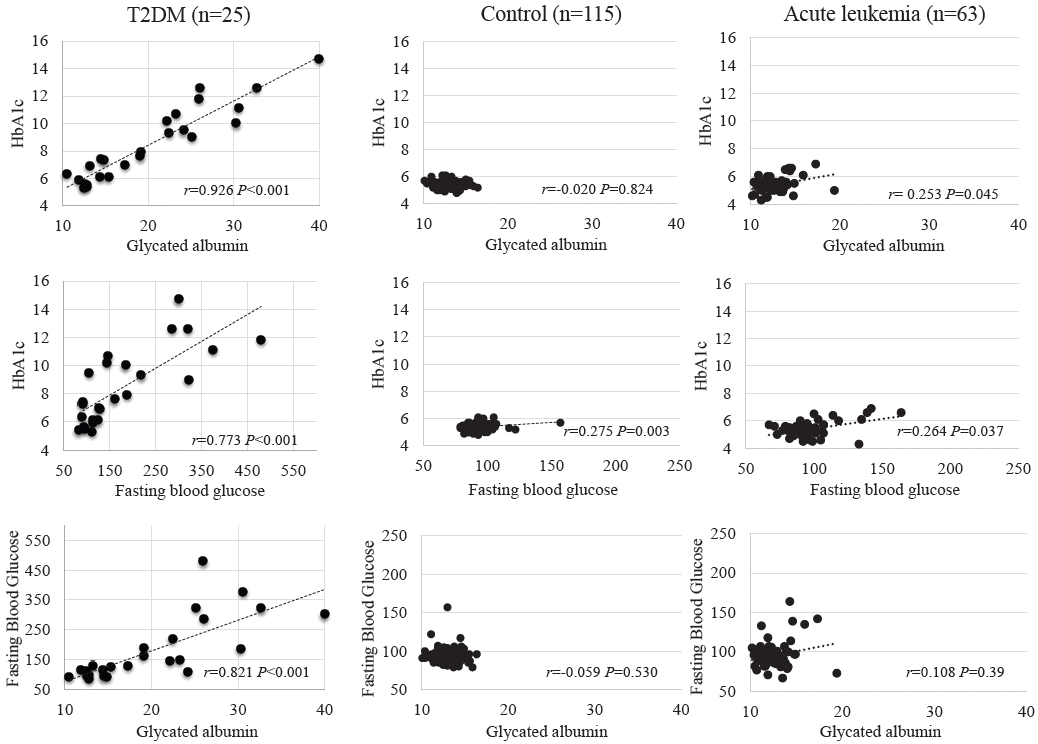
Fig. 3.
Correlation between the BMI z-score and the glycated albumin, HbA1c, and fasting blood glucose levels in each study group. HbA1c, glycated hemoglobin; T2DM, type 2 diabetes melliuts; BMI, body mass index.
Table 1.
Characteristics of the study participants
| Characteristic | Total (n=203) | T2DM (n=25) | Control (n=115) | Acute Leukemia (n=63) | P-value | ||
|---|---|---|---|---|---|---|---|
| Age (yr) | 11 (9–13) | 15 (13–17) | 10 (8.0–12.0) | 11 (8–14) | <0.001*,‡ | ||
| Male sex | 117 (57.6) | 11 (44) | 78 (67.8) | 28 (44.1) | 0.006*,† | ||
| BMI z-score | 0.69 (-0.52 to 2.16) | 2.18 (1.42–2.98) | 0.69 (-0.49 to 1.99) | 0.17 (-0.87 to 1.54) | <0.001*,‡ | ||
| Obesity | 68 (33.5) | 15 (60) | 37 (32) | 16 (25) | 0.008*,‡ | ||
| Height z-score | -0.08 (-0.91 to 0.69) | 0.89 (0.58–1.63) | -0.25 (-0.94 to 0.57) | -0.28 (-1.05 to 0.32) | <0.001*,‡ | ||
| Weight z-score | 0.43 (-0.62 to 1.78) | 2.49 (1.44–3.04) | 0.38 (-0.58 to 1.73) | 0.03 (-1.27 to 1.36) | <0.001*,‡ | ||
| Age at diagnosis (years) | - | 14 (12–15) | - | 6 (4.00–11.0) | |||
| Diagnosis | - | - | - | Lymphoblastic | 48 (76) | ||
| Myeloid | 15 (24) | ||||||
| Treatment | Insulin | 2 (8) | - | Transplantation | 33 (52.4) | ||
| Metformin | 14 (56) | - | Cumulative glucocorticoid dose (g) | 4,910 (1,870–7,090) | |||
| Combination | 9 (36) | - | Cumulative L-asparaginase dose (KIU) | 59,100 (50,100–81,100) | |||
Table 2.
Biochemical data of the study participants
| Variable | T2DM (n=25) | Control (n=115) | Acute leukemia (n=63) | P-value |
|---|---|---|---|---|
| Glycated albumin (%) | 19.1 (13.2–25.2) | 13.0 (12.1–13.9) | 12.3 (11.5–13.4) | <0.001*,†,‡ |
| Albumin (g/dL) | 4.8 (4.6–4.9) | 4.7 (4.6–4.9) | 4.7 (4.5–4.9) | 0.105 |
| HbA1c (%) | 7.6 (6.1–10.2) | 5.4 (5.3–5.6) | 5.3 (5.1–5.6) | <0.001*,‡ |
| FBG (mg/dL) | 129 (106–219) | 92.0 (88.5–96.0) | 92.0 (87.5–101.0) | <0.001*,‡ |
| WBC (109/L) | 7.7 (5.8–8.8) | 6.7 (5.8–7.8) | 5.9 (4.6–7.5) | 0.003†,‡ |
| Hb (g/dL) | 14.3 (13.4–14.9) | 13.5 (12.9–14.3) | 12.9 (12.1–13.9) | <0.001*,†,‡ |
| Platelet (109/L) | 295 (260–359) | 318 (281–361) | 252 (210–314) | <0.001†,‡ |
| HOMA-IR | 5.5 (4.4–11.6) | 3.5 (2.1–6.1) | 3.5 (2.1–6.4) | 0.001*,‡ |
| C-peptide (ng/mL) | 3.3 (2.3–3.4) | 2.5 (1.9–3.5) | 2.4 (1.7–4.1) | 0.412 |
| HDL-cholesterol (mg/dL) | 43 (38–55) | 55 (46–65) | 48 (43–58) | <0.001* |
| Triglycerides (mg/dL) | 133 (76–208) | 80 (53–129) | 112 (67–183) | 0.002*,† |
| Total protein (g/dL) | 7.4 (7.15–7.9) | 7.1 (6.9–7.4) | 6.9 (6.4–7.3) | 0.001*,‡ |
Table 3.
Factors related to HbA1c elevation




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download