Abstract
Objective
Advancements in AVM surgical techniques for cerebral arteriovenous malformation (AVM) underscore its efficacy. Our research aims to showcase the positive outcomes of treating low-grade AVMs surgically, focusing on safety and effectiveness.
Methods
We retrospectively reviewed 55 patients (36 males and 19 females; average age 37.4 years) with Spetzler-Martin (S-M) grade 1 and 2 AVMs who underwent surgical resection between January 2009 and December 2022.
Results
In our study, 55 patients with S-M grade 1 and 2 AVMs underwent surgical resection, evenly divided between grades 1 (50.9%) and 2 (49.1%). Intracranial hemorrhage was the primary symptom in 74.5% of cases. Pre-operative Glasgow coma scale (GCS) scores revealed 69.1% of patients scored above 13, with 18% below 8. Successful resection was achieved in 87.3%. Postoperatively, 95.5% of ruptured and 90.9% of unruptured AVM patients showed lower or same modified Rankin scale scores. Poorer outcomes were significantly linked to lower GCS scores and intranidal/flow-related aneurysms through multivariate logistic regression. Postoperative seizures noted in nine patients, were exclusive to the ruptured AVM group.
Conclusion
Our findings indicate surgical resection as a beneficial treatment for low-grade AVMs, yielding high cure rates and positive functional outcomes in both ruptured and unruptured cases. Preoperative GCS scores and the presence of associated aneurysms are predictive of postoperative functional status. Additionally, managing postoperative seizures effectively is key to enhancing prognosis.
Surgical resection is widely regarded as a preferred approach for treating cerebral arteriovenous malformations (AVMs) due to its demonstrated high cure rate, low complication rate, and the immediacy of its treatment effectiveness, as evidenced by current surgical results with low-grade brain AVMs [14]. Improvements in surgical outcomes over time can be attributed to several factors : the development of grading systems to select optimal candidates, the recognition of AVM subtypes (which has aided in understanding AVM anatomy) [2], advances in tools such as bipolar forceps and microclips for effective occlusion or embolization of feeding arteries [14], and the progression of approaches and surgical strategies that facilitate safe AVM surgery [8].
Such advancements in AVM surgery stand in contrast to the findings of the ARUBA (a randomized trial of unruptured brain arteriovenous malformations) trial [11]. The ARUBA trial demonstrated the superiority of medical management over interventional therapy in terms of mortality or stroke among patients with unruptured AVMs tracked over 33 months [11]. However, the premature termination of the ARUBA study after interim results were analyzed has faced significant criticism [4].
Despite receiving multiple criticisms, the ARUBA yielded a new standard for medical treatment, diminishing the perceived importance of surgical intervention. We aimed to demonstrate, through 14 years of accumulated data, that surgery does not lead to adverse outcomes. The ARUBA study focused on unruptured AVMs, but we investigated low-grade AVMs, regardless of rupture status, to showcase the positive outcomes of surgical treatment. In this study, we selected surgical patients who had Spetzler-Martin (S-M) grade 1 and 2 AVMs and analyzed the safety and efficacy of the procedures.
The study was conducted following a review by the Institutional Review Board of Chonnam National University Hospital (CNUH-2022-203).
We conducted a retrospective review analyzing data of patients managed between January 2009 and December 2022 at Chonnam National University Hospital. The review included a total of 55 patients with S-M grade 1 and 2 AVMs who underwent surgery, selected from 407 overall AVM cases. These patients were treated regardless of rupture status and under the guidance of three surgeons at a single center.
Patients’ preoperative conditions were evaluated using the Glasgow coma scale (GCS), supplemented by medical records and database records. Functional outcomes were assessed using the modified Rankin scale (mRS), dichotomized into “good” (mRS 0–1) or “poor” (mRS 2–6), which were obtained both before surgery and at a 90-day follow-up observation period for each patient. Baseline functional status was evaluated at outpatient visits or hospital admissions prior to treatment, while subsequent information was gathered during postoperative outpatient visits or hospital admissions. Postoperative vascular angiographic results were determined by neuro-interventional radiologists, with complete removal defined as the absence of residual nidi on vascular angiography. The good angiographic outcome was defined as the ratio of patients with complete removal among all those who underwent postoperative angiography.
Statistical analysis was performed using Stata/SE 16.1 (StataCorp, College Station, TX, USA). For all categorical variables, cross-tabulation was generated, and Fisher’s exact test, the chi-square test, and linear-by-linear association were employed. Continuous variables were compared using the Mann-Whitney U test. Multivariate analysis was also conducted. Characteristics with p-values less than 0.05 were considered statistically significant.
Within our cohort of 407 patients, exclusions were made for several reasons : 159 patients were excluded due to high S-M grades (≥3), 25 for receiving non-surgical treatments despite low S-M grades (<3), 26 due to loss to follow-up (including treatment refusal or hospital transfer), and 142 underwent observation, resulting in 55 patients who proceeded with surgical interventions. This subgroup comprised 11 patients with unruptured AVMs—eight with grade 1 lesions and three with grade 2 lesions—and 44 with ruptured AVMs, including 20 with grade 1 lesions and 24 with grade 2 lesions (Fig. 1).
Fig. 2 illustrates the surgical removal process for a ruptured AVM, showing preoperative imaging, the surgical approach, and postoperative outcomes confirming AVM elimination. Fig. 3 showcases the excision of an unruptured AVM, with initial diagnostics, detailed surgical steps, and evidence of complete removal through postoperative imaging.
Table 1 delineates the fundamental characteristics of the 55 surgical interventions. Overall, the mean age was 37.40±18.56 years, with a slight male preponderance (65.5%). The most common AVM locations were the frontal lobe (30.9%) and the temporal lobe (30.9%). AVMs were more frequently located on the right side (70.9%), and 31.0% of the total cohort had accompanying nidal or flow-related aneurysms. S-M grades were nearly equally distributed, with 50.9% in grade 1 and 49.1% in grade 2.
Intracranial hemorrhage (74.5%) was the most common initial clinical presentation in the overall cohort, accompanied by seizures and headaches. The preoperative GCS scores were above 13 in 69.1% of the overall cohort and below 8 in 18%. One patient (1.8%) underwent preoperative radiosurgery.
All patients underwent postoperative vascular angiography to confirm residual AVMs. Table 2 shows that residual AVMs were not observed in 48 patients (87.3%), confirming complete AVM resection. Among these 48 patients without any remnant AVM tissue, 37 had ruptured AVMs, and the others had unruptured AVMs. Remnant AVMs were identified in four patients (7.3%) during postoperative vascular angiography, all of whom had ruptured AVMs. The remaining three patients with ruptured AVMs either refused postoperative vascular angiography (n=1) or died (n=2). This result suggests a good angiographic outcome of 92.3% (48/52).
Although there were two deaths in this study, the mortality rate is reported as 0%. The deceased patients did not die directly due to AVM surgery; instead, they passed away due to their conditions upon hospital admission, already in critical condition due to AVM rupture.
As summarized in Table 2, in the ruptured AVM cohort, 37 out of 44 patients (84.1%) achieved complete angiographic obliteration, while four patients (9.1%) had residual AVM, and three patients (6.8%) did not undergo postoperative angiographic evaluation. Conversely, all 11 patients (100.0%) with unruptured AVMs achieved complete angiographic obliteration, with no residual AVMs or missing postoperative studies. Overall, the cohort exhibited a high complete obliteration rate of 87.3% (48 out of 55 patients), with 7.3% (four patients) having residual AVMs and 5.4% (three patients) not undergoing postoperative angiographic evaluation. These findings underscore the efficacy of surgical resection in achieving favorable angiographic outcomes, particularly in unruptured AVMs, which demonstrated a 100.0% rate of complete obliteration.
Regarding functional outcomes after surgery (Table 2), in the ruptured AVM group, 26 patients (59.1%) had favorable outcomes (mRS 0–1), while two patients died. In the unruptured AVM group, all 11 patients (100.0%) had favorable outcomes (mRS 0–1). Among the patients with ruptured AVMs, 42 (95.5%) had either improved or unchanged mRS scores, while among the patients with unruptured AVMs, 10 (90.9%) had either improved or unchanged mRS scores. In a single case of an unruptured AVM patient, despite the exacerbation of the mRS score from 0 to 1, the patient was classified as achieving a favorable outcome upon assessment based on outcome criteria.
In the univariate analysis of factors related to favorable (mRS 0–1) versus poor (mRS ≥2) functional outcomes (Table 3), the preoperative GCS score was identified as a significant predictor. Specifically, a GCS score of 9–12 was associated with an odds ratio of 7.11 (p=0.026), while a GCS score <9 exhibited an odds ratio of 21.33 (p=0.001). Additionally, accompanying aneurysms (intranidal or flow-related) were significantly associated with poor outcomes. Notably, factors such as AVM location, S-M grade itself, and components of the S-M grade (size, eloquence of adjacent brain parenchyma, venous drainage pattern) were not significantly associated with functional outcomes. Furthermore, multivariate logistic regression analysis on the four variables with a p-value of ≤0.1 in the univariate logistic regression analysis revealed significant associations between groups with low GCS scores (9–12 and <9) and accompanying intranidal/flow-related aneurysms.
After surgical AVM removal, 12 patients (21%) manifested new symptoms. Among them, nine developed seizures, all of whom were in the ruptured AVM group. Additionally, one patient in the ruptured AVM group developed quadrantanopsia, while another developed homonymous hemianopsia. In the unruptured AVM group, only one patient exhibited new symptoms, which were manifestations of motor aphasia.
Surgical complications were experienced by four patients (7.3%), all in the ruptured AVM group. These complications included subdural hemorrhage and an epidural abscess at the surgical site. Furthermore, two patients experienced cerebrospinal fluid leakage. Successful revision procedures were performed on all patients who developed surgical complications.
This study’s findings underscore the efficacy of surgical treatment for low-grade AVMs deemed to require intervention. Resection is generally considered the first-line approach for most AVMs in resectable areas, complemented by adjunctive embolization or radiosurgery for AVMs situated in deep and inaccessible regions [14].
Patients, with a mean age of 37.4 years, were carefully selected to optimize outcomes. Among those who underwent surgery, a good angiographic outcome of 92.3% was achieved. Postoperatively, neurological deterioration was limited to three individuals (5%), while favorable outcomes (mRS 0–1) were observed in 52 patients (95%) overall, including 42 patients with ruptured AVMs and 10 with unruptured AVMs. These surgical outcomes are consistent with findings from other studies [4,10,12,17].
Endovascular embolization is frequently employed to shrink large AVMs, improve the safety of surgery, or prepare AVMs for radiosurgery [6]. Some small AVMs have been successfully treated with embolization alone [6]. Onyx (ethylene-vinyl alcohol copolymer) is a widely used embolic agent, favored for its lower adhesive properties and slower polymerization compared with NBCA (n-butyl cyanoacrylate), offering distinct advantages [7]. In a review article reporting on 1297 patients primarily with low-grade AVMs, the average rates of endovascular morbidity and mortality were calculated to be 6.2% and 1.6%, respectively, with an average obliteration rate of 29% and a postoperative or delayed hemorrhage rate of 8.0% [14]. A prospective study of 224 patients treated endovascularly for low-grade AVMs found 205 patients (92%) to be completely free of brain AVMs, with 139 patients (62.1%) cured in a single endovascular treatment session; 85 patients (38%) required additional endovascular procedures [1]. Among 19 patients with residual AVMs after endovascular treatment, eight (3.6%) underwent surgical completion, while nine (4%) received radiosurgical interventions [1]. Overall favorable outcomes (mRS 0–2) were reported for 179 patients (80%), but the mRS scores of 13 patients (6%) decreased relative to their pretreatment scores [1]. Twelve patients (5%) exhibited permanent neurological deficits after treatment, and the mortality rate was 0.4% (one patient) [1]. Another study of 109 patients showed that 89.9% achieved complete exclusion after endovascular treatment sessions, with 59.6% achieving this in a single session. At the 6-month follow-up, 97.2% had favorable outcomes, with only 4.6% experiencing transient neurological deficits and 0.9% having permanent deficits [15]. These studies suggest that endovascular treatment can be safe and effective. However, it often requires multiple sessions for successful outcomes and may necessitate additional therapies, such as surgery or radiosurgery. Nevertheless, surgery was associated with low recurrence rates during follow-up, with few patients requiring additional treatment among those with low-grade AVMs.
Low-grade AVMs are ideal candidates for radiosurgery owing to their small target volumes and high obliteration rates; however, the latency period of 2 to 3 years between treatment and obliteration presents a risk window for AVM hemorrhage and associated complications [13]. A review of 1051 patients with low-grade AVMs revealed that 7.2% experienced posttreatment hemorrhage, with morbidity and mortality rates of 6.5% and 1.2%, respectively [14]. The radiosurgical obliteration rate of 75.2% was significantly lower than the surgical obliteration rate.
Furthermore, embolization of AVMs often precedes radiosurgery, but this sequence can compromise the outcomes of radiosurgical procedures. Indeed, obliteration rates associated with partial embolization before radiosurgery have been shown to be notably lower than those with untreated malformations [16]. This may be due to the occlusion of smaller arterial feeders triggering vascular remodeling in response to ischemia and partial embolization inducing the recruitment of new vascular subsystems, thereby reducing the effectiveness of radiosurgery [16]. Recently researched studies indicate that microsurgery for low-grade AVMs showed higher obliteration rates (96% vs. 57%, p<0.001) and better early post-treatment hemorrhage-free survival compared to radiosurgery, with similar long-term functional outcomes and complication rates [5]. Therefore, despite advancements in endovascular and radiosurgical techniques, surgery remains a sufficiently effective treatment option, offering high obliteration rates, a favorable risk profile, and reliable protection against hemorrhage in the context of low-grade AVMs.
Previous studies have not clearly established the association between preoperative GCS scores and the postoperative functional outcomes of patients with low-grade AVMs. Our single-center study specifically focused on this aspect, finding that lower preoperative GCS scores were associated with poorer functional outcomes, and the presence of an accompanying aneurysm was associated with even worse functional outcomes. This suggests that surgical treatment may be beneficial not only in cases with established indications for surgery, such as AVM rupture or accompanying neurological symptoms, but also in cases with poorer prognostic factors, such as low preoperative GCS scores and low-grade AVMs with accompanying aneurysms.
Moreover, the most common postoperative complication in this study was seizures (incidence, 16.4%). A prospective study of 440 patients with supratentorial AVMs who underwent microsurgical resection determined a postoperative seizure rate of 3% in patients without preoperative seizures [3], which was lower than the rate observed in our study. Postoperative seizures were considered a risk factor, particularly in cases involving the temporal lobe or associated with intracranial hypertension, regardless of preoperative seizure status. However, another population-based study involving 229 patients found no difference in the 5-year risk of seizures between surgical AVM management and conservative management [9]. Therefore, rather than avoiding surgery based solely on the risk of postoperative seizures, appropriate postoperative seizure management may lead to better outcomes.
Additionally, perioperative complications are comparable to those associated with standard craniotomy. However, when an AVM ruptures without surgical intervention and neurological symptoms occur, there may be lasting sequelae. Therefore, the decision for surgery should not be based solely on the presence of symptoms at the time of AVM discovery; proactive surgical intervention can lead to better outcomes.
Surgical resection should be regarded as a viable treatment option for low-grade AVMs. Both ruptured and unruptured cases exhibited high rates of good angiographic outcomes and excellent functional outcomes. The preoperative GCS score and associated aneurysm status may serve as important predictive factors for postoperative functional outcomes. Moreover, effective postoperative seizure management is essential for a favorable prognosis.
Notes
Informed consent
Informed consent was obtained from all individual participants included in this study.
ACKNOWLEDGMENTS
This study was supported by a grant (BCRI24072) from the Chonnam National University Hospital Biomedical Research Institute.
References
1. Baharvahdat H, Blanc R, Fahed R, Smajda S, Ciccio G, Desilles JP, et al. Endovascular treatment for low-grade (Spetzler-Martin I-II) brain arteriovenous malformations. AJNR Am J Neuroradiol. 40:668–672. 2019.
2. Davies JM, Kim H, Young WL, Lawton MT. Classification schemes for arteriovenous malformations. Neurosurg Clin N Am. 23:43–53. 2012.

3. Englot DJ, Young WL, Han SJ, McCulloch CE, Chang EF, Lawton MT. Seizure predictors and control after microsurgical resection of supratentorial arteriovenous malformations in 440 patients. Neurosurgery. 71:572–580. discussion 580. 2012.
4. Feghali J, Huang J. Updates in arteriovenous malformation management: the post-ARUBA era. Stroke Vasc Neurol. 5:34–39. 2019.
5. Gami A, Feghali J, Rapaport S, Sattari SA, Yang W, Tamargo RJ, et al. Microsurgical resection versus stereotactic radiosurgery for low-grade intracranial arteriovenous malformations: a 27-year institutional experience. J Clin Neurosci. 94:209–215. 2021.

6. Gobin YP, Laurent A, Merienne L, Schlienger M, Aymard A, Houdart E, et al. Treatment of brain arteriovenous malformations by embolization and radiosurgery. J Neurosurg. 85:19–28. 1996.
7. Guimaraes M, Wooster M. Onyx (ethylene-vinyl alcohol copolymer) in peripheral applications. Semin Intervent Radiol. 28:350–356. 2011.

8. Hamilton MG, Spetzler RF. The prospective application of a grading system for arteriovenous malformations. Neurosurgery. 34:2–6. discussion 6-7. 1994.

9. Josephson CB, Bhattacharya JJ, Counsell CE, Papanastassiou V, Ritchie V, Roberts R, et al. Seizure risk with AVM treatment or conservative management: prospective, population-based study. Neurology. 79:500–507. 2012.

10. Link TW, Winston G, Schwarz JT, Lin N, Patsalides A, Gobin P, et al. Treatment of unruptured brain arteriovenous malformations: a singlecenter experience of 86 patients and a critique of the a randomized trial of unruptured brain arteriovenous malformations (ARUBA) trial. World Neurosurg. 120:e1156–e1162. 2018.
11. Mohr JP, Parides MK, Stapf C, Moquete E, Moy CS, Overbey JR, et al. Medical management with or without interventional therapy for unruptured brain arteriovenous malformations (ARUBA): a multicentre, nonblinded, randomised trial. Lancet. 383:614–621. 2014.
12. Moon K, Levitt MR, Almefty RO, Nakaji P, Albuquerque FC, Zabramski JM, et al. Safety and efficacy of surgical resection of unruptured lowgrade arteriovenous malformations from the modern decade. Neurosurgery. 77:948–952. discussion 952-953. 2015.

13. Peschillo S, Caporlingua A, Colonnese C, Guidetti G. Brain AVMs: an endovascular, surgical, and radiosurgical update. ScientificWorldJournal. 2014:834931. 2014.

14. Potts MB, Lau D, Abla AA, Kim H, Young WL, Lawton MT, et al. Current surgical results with low-grade brain arteriovenous malformations. J Neurosurg. 122:912–920. 2015.

15. Razavi SAS, Mirbolouk MH, Gorji R, Ebrahimnia F, Sasannejad P, Zabihyan S, et al. Endovascular treatment as the first-line approach for cure of low-grade brain arteriovenous malformation. Neurosurg Focus. 53:E8. 2022.
16. Rubin BA, Brunswick A, Riina H, Kondziolka D. Advances in radiosurgery for arteriovenous malformations of the brain. Neurosurgery 74 Suppl. 1:S50–S59. 2014.

17. Rutledge WC, Abla AA, Nelson J, Halbach VV, Kim H, Lawton MT. Treatment and outcomes of ARUBA-eligible patients with unruptured brain arteriovenous malformations at a single institution. Neurosurg Focus. 37:E8. 2014.
Fig. 1.
Patient selection process flowchart. AVM : arteriovenous malformation, S-M : Spetzler-Martin.
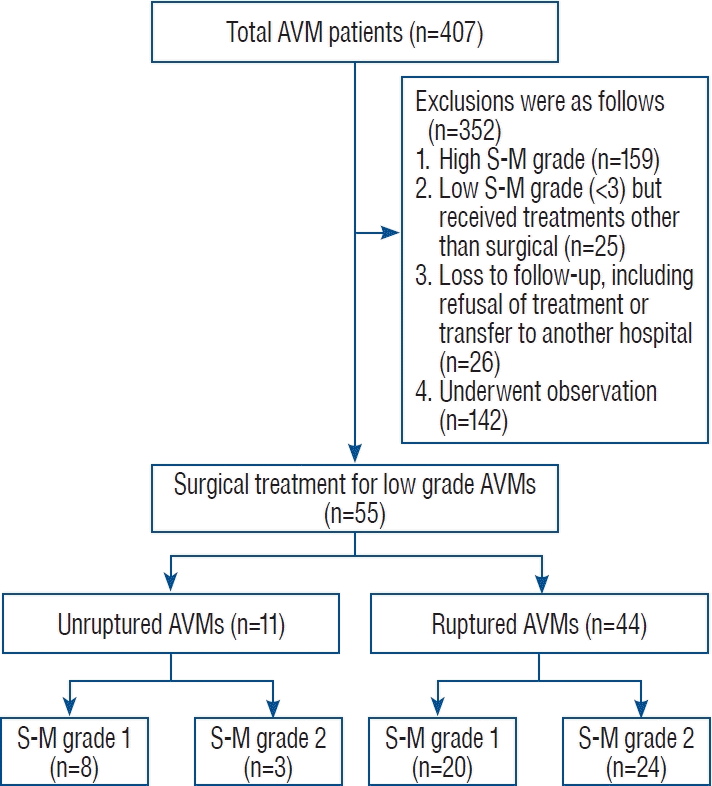
Fig. 2.
Surgical removal for ruptured arteriovenous malformation (AVM) case. A : Pre-operative computed tomography scan illustrating a right frontoparietal hematoma. B : Pre-operative digital subtraction angiogram (DSA) from the right internal carotid artery reveals the AVM as the source of hemorrhage, slated for surgical excision. C : Post-operative DSA confirming the AVM’s absence, indicative of successful surgical removal. D and E : Detailed surgical approach involving microsurgical removal of the AVM via navigated craniotomy and meticulous excision, including clipping of the feeding artery. F : The extracted surgical specimen, showcasing the completely resected AVM.
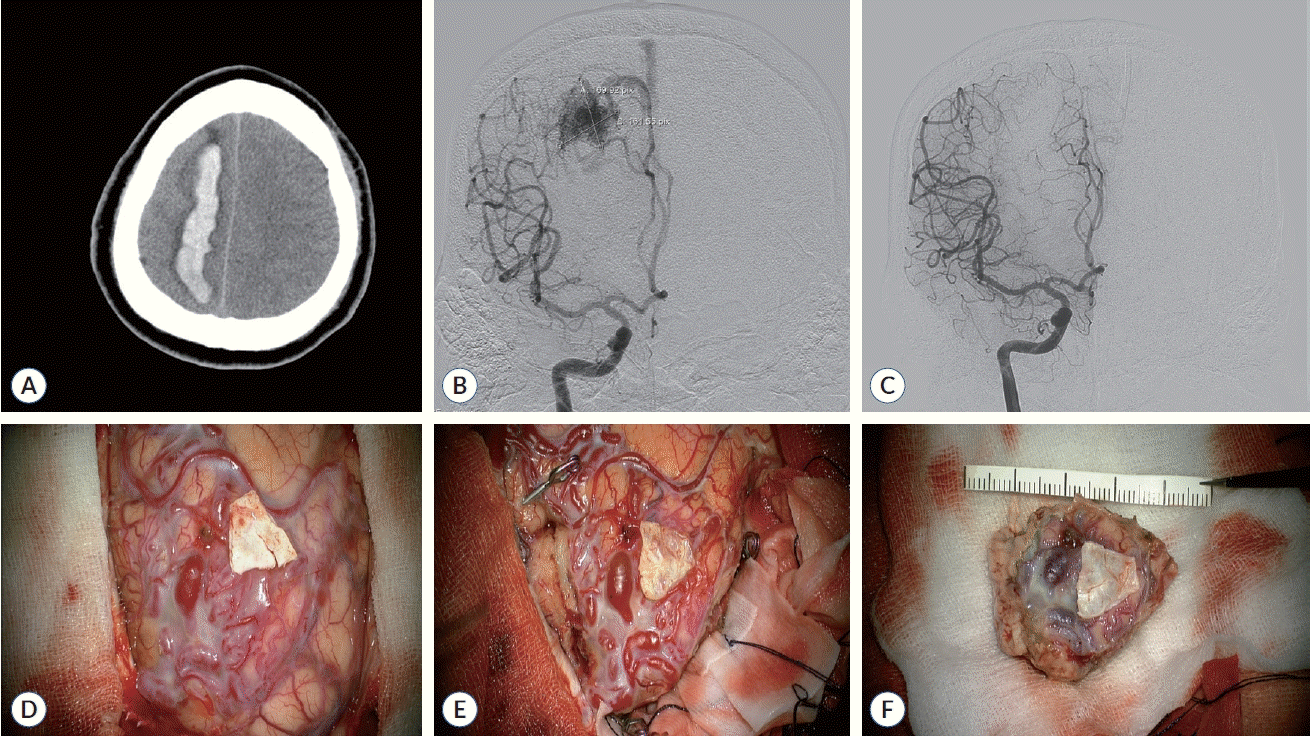
Fig. 3.
Surgical removal for unruptured arteriovenous malformation (AVM) case. A : Pre-operative gadolinium-enhanced axial T1-weighted magnetic resonance image depicts an unruptured right frontal AVM. B : Pre-operative digital subtraction angiography (DSA) from the right internal carotid artery identifies the AVM, targeted for surgical removal. C : Post-operative DSA confirms the AVM’s absence, indicating successful surgical eradication. D and E : Description of the surgical technique involving microsurgical removal of the AVM via navigated craniotomy and precise excision, with clipping of the feeding artery. F : The surgical specimen, demonstrating the completely excised AVM.
Table 1.
Characteristics of low-grade AVM patients treated surgically
Table 2.
Angiographic and functional outcome of low-grade AVM patients undergone surgery
Table 3.
Surgical results with low-grade AVMs
|
mRS outcome |
Univariate logistic regression |
Multivariate logistic regression |
||||
|---|---|---|---|---|---|---|
| Good (0–1) | Poor (2–6) | OR (95% CI) | p-value* | OR (95% CI) | p-value† | |
| Number of patients | 37 | 18 | ||||
| Sex | ||||||
| Female | 13 (35.1) | 6 (33.3) | 1 (reference) | |||
| Male | 24 (64.9) | 12 (66.7) | 1.08 (0.33–3.56) | 0.895 | ||
| Age (years) | 36.05±19.53 | 37.44±17.28 | 1.000 (0.97–1.04) | 0.794 | ||
| AVM site | ||||||
| Right | 28 (75.7) | 9 (50.0) | 1 (reference) | 1 (reference) | ||
| Left | 9 (24.3) | 9 (50.0) | 3.11 (0.95–10.23) | 0.062 | 3.10 (0.54–17.90) | 0.205 |
| S-M grade | ||||||
| I | 20 (54.1) | 8 (44.4) | 1 (reference) | |||
| II | 17 (45.9) | 10 (55.6) | 1.47 (0.47–4.56) | 0.504 | ||
| Size | ||||||
| 1 (<3 cm) | 30 (81.1) | 12 (66.7) | 1 (reference) | |||
| 2 (3–6 cm) | 7 (18.9) | 6 (33.3) | 2.14 (0.60–7.70) | 0.243 | ||
| Eloquence of adjacent brain parenchyma | ||||||
| 0, non-eloquent | 28 (75.7) | 14 (77.8) | 1 (reference) | |||
| 1, eloquent | 9 (24.3) | 4 (22.2) | 0.89 (0.23–3.40) | 0.863 | ||
| Venous drainage | ||||||
| 0, superficial | 36 (97.3) | 17 (94.4) | 1 (reference) | |||
| 1, deep | 1 (2.7) | 1 (5.6) | 2.12 (0.12–35.93) | 0.603 | ||
| Rupture | ||||||
| Unruptured | 11 (29.7) | 0 (0.0) | ||||
| Ruptured | 26 (70.3) | 18 (100.0) | - | - | ||
| GCS scale | ||||||
| 13–15 | 32 (86.5) | 6 (33.3) | 1 (reference) | 1 (reference) | ||
| 9–12 | 3 (8.1) | 4 (22.2) | 7.11 (1.26–40.21) | 0.026 | 16.74 (1.96–142.69) | 0.010 |
| <9 | 2 (5.4) | 8 (44.5) | 21.33 (3.60–126.25) | 0.001 | 24.86 (2.87–215.35) | 0.004 |
| Associated aneurysm | ||||||
| None | 29 (78.4) | 9 (50.0) | 1 (reference) | 1 (reference) | ||
| Nidal or flow related | 8 (21.6) | 9 (50.0) | 3.63 (1.08–12.17) | 0.037 | 11.14 (1.65–75.15) | 0.013 |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download