Abstract
Objective
The use of reconstructive treatment with a double-overlapping stents has proven to be effective and safe in the current treatment of intracranial vertebral artery dissecting aneurysms (VADAs). We employed a combination of overlapping stents, using low-profile visualized intraluminal support (LVIS) within the Enterprise stent. This combination was chosen to minimize the outward bulging of the inner LVIS by overlapping it with the Enterprise stent while maintaining flow diversion and stability. This study aimed to evaluate the clinical and angiographic outcomes following the use of double-overlapping stents with LVIS within the Enterprise stent for the treatment of VADAs.
Methods
From March 2016 to January 2022, total 28 patients with unruptured VADAs were treated with the double-overlapping stent technique using LVIS within an Enterprise stent in our institute. The Enterprise stent was deployed first, followed by the LVIS stent. Patient clinical and angiographic characteristics, procedural complications, and follow-up outcomes were retrospectively reviewed.
Results
All 28 patients (18 males and 10 females) were successfully treated with double-overlapping stent deployment. There were no procedural complications or new neurological deficits in any patient. Of the 28 patients, four VADAs had posterior inferior cerebellar artery involvement. Procedure-related parent artery occlusion did not occur during the angiographic follow-up conducted 6 to 12 months after the procedure. Out of 28 patients, 24 showed complete healing, three had focal residual stenosis or dilatation with residual sac and only one had a residual dissecting flap with aneurysm. All patients, including the four patients, did not require any additional procedures. The postoperative modified Rankin scale scores were 0–1 for all patients.
Conclusion
A double-overlapping stent, with a flow-diversion effect, is a safe and effective treatment for patients with VADAs. In particular, when using the LVIS stent within an Enterprise stent, it minimizes the bulging of the inner LVIS stent while maintaining flow diversion and stability. Therefore, both can be effectively utilized as overlapping stents.
Intracranial vertebral artery dissecting aneurysms (VADAs) are rare vascular diseases that can lead to subarachnoid hemorrhage (SAH) or acute ischemic stroke. The incidence of VADAs is unknown, but it is reported to be the most common cervical artery dissection in East Asia [10]. In most series, the vertebral artery (VA) (intradural segment) is the most common site of intracranial artery dissection. VADAs can lead to devastating events that can cause serious neurological deficits in individuals. They can occur in people of all ages and are known to be a major cause of strokes and SAH, especially in young and middle-aged people [23]. To prevent thromboembolic complications in symptomatic, unruptured VADA patients, primary conservative treatment is typically initiated, which primarily involves anticoagulation or antiplatelet therapy.
Nevertheless, when there is no response to the initial medication therapy and recurrent ischemia occurs, and when there are progressive vascular morphological changes observed on repeated radiologic imaging, or when the extent and size of the dissecting aneurysm result in mass effect leading to the onset or exacerbation of neurological symptoms, additional treatment strategies need to be considered. Reconstructive treatment, which preserves the parent artery flow, has recently been performed as a major treatment for unruptured VADAs and has been proven to be effective in previous studies [1,2,5,6,11,22]. Traditionally, VADA patients are treated with reconstructive therapy using single stent-assisted coil (SAC) embolization, overlapping SAC embolization, patent artery occlusion, overlapping stent implantation, and flow diverter (FD) implantation [17]. SAC embolization is technically difficult in the case of posterior inferior cerebellar artery (PICA) involvement, fusiform and circumferential dilatation among some VADAs, so stent only deployment can be an alternative treatment option. In particular, many treatments have recently been conducted to increase the flow diversion effect by overlapping stent, and the effect has been proved. We employed a combination of overlapping stents, using low-profile visualized intraluminal support (LVIS) within the Enterprise stent. This combination was chosen to minimize the outward bulging of the inner LVIS by overlapping it with the Enterprise stent while maintaining flow diversion and stability.
This study aimed to evaluate the clinical and angiographic outcomes of the use of a double-overlapping stent technique with an LVIS within an Enterprise stent for the treatment of VADAs.
This study was performed in accordance with the Declaration of Helsinki and its amendments. The study was approved by the Institutional Review Board (IRB) of Ajou University Hospital (ethics approval number : IRB-DB-2022-517). The requirement for patient consent was waived owing to the retrospective nature of the study.
The treatment indication for VADAs is when the first patient presents with neurological symptoms. If symptoms are present but mild, and the dissection is not extensive, with satisfactory flow through the contralateral dominant VA, medical therapy may be considered as a priority. Treatment may be considered, especially in cases where patients have acute ischemia, and there is a high likelihood of recurrent ischemic events. Secondly, intervention treatment should be initiated if there is evidence of progression in the dissection lesion on serial computed tomography (CT) angiography follow-up images or if there is a worsening pattern observed in neurological symptoms. In addition, for patients who find it challenging to take antiplatelet agents over an extended period and experience persistent symptoms, early initiation of treatment could be considered. This approach may involve maintaining antiplatelet therapy initially and considering discontinuation later on.
Our study was conducted retrospectively on a patient cohort admitted to our institution from March 2016 to January 2022. All patients included in the study cohort underwent double-overlapping stent deployment using LVIS within an Enterprise stent.
A total of 43 patients, who underwent overlapping stent placement for VADAs, were enrolled. Five patients, who did not undergo follow-up imaging or had a follow-up period of less than 6 months, were excluded. Of the remaining 38 patients, 28 were treated with double-overlapping stents, using the LVIS stent deployed within an Enterprise stent. We excluded the remaining 10 patients from this study because of using different types of overlapping stents (seven patients for Enterprise; Cordis Neurovascular, Miami Lakes, FL, USA) within Enterprise stent and two patients for Solitaire AB stent (ev3 Neurovascular, Irvine, CA, USA) within enterprise and one patient for LVIS (MicroVention, Tustin, CA, USA) within LVIS stent). All patients who underwent overlapping stent placement had unruptured VADAs. The demographic and clinical characteristics of the patients are shown in Table 1. All patients underwent digital subtraction angiography (DSA) and rotational angiography with three-dimensional reconstruction to identify the lesion and parent artery anatomy. The sizes, shapes, and locations of the VADAs were assessed, as well as major arterial branches and collaterals. Furthermore, the extent of dissection of the VA and involvement of the PICA were also examined. Along with the patient’s clinical presentation, a pearl and string sign or fusiform dilation and string sign (isolated irregular navigation) observed on DSA was considered indicative of PICA involvement in VADAs. In addition, high-resolution magnetic resonance imaging (MRI) was performed on all patients, and all T1-enhanced imaging findings were comprehensively checked for reflecting vessel wall enhancement and the double lumen sign, which are characteristic findings of VADA.
Follow-up DSA was performed between 6 and 12 months after stenting, and clinical follow-up was performed once every 6 months after the procedure in all patients. After DSA, follow-up was conducted using CT angiography. Radiologic outcomes were evaluated using the modified Raymond grade, which includes grade 1; complete occlusion, grade 2; residual neck, and grade 3; residual aneurysm. Clinical outcomes were assessed using the modified Rankin score (mRS) based on the last clinical follow-up date. Post-procedural complications were assessed for immediate and delayed occurrences (>1 month).
Before the procedure, all 28 patients were administered orally dual antiplatelet drugs (100 mg/day aspirin; 75 mg/day, clopidogrel) with premedication for a week, followed by stent procedures. Anticoagulation was started during the procedure to prevent in-stent thrombosis of 1000 or 2000 units heparin via intravenous injection as a bolus. After the procedure, heparin fluid was used in the intensive care unit for 24 hours routinely to maintain activated partial thromboplastin time of 60 to 80 seconds. All patients underwent a platelet function test before the double-overlapping stents procedure, and regardless of the results of the resistance test, they maintained the initial dual antiplatelet therapy. The patients continued the dual antiplatelet regimen for over 12 months, and if, based on the physician’s judgment, the follow-up images indicated the healing process and stable condition, they transitioned to and maintained a protocol with aspirin monotherapy. There were no bleeding or ischemic events during the follow-up period.
Stent deployment procedure was performed under general anesthesia for all patients. A 6 F sheath was inserted to the right femoral artery for the puncture site and a 6 F guiding catheter (Envoy; Cordis, Miami Lakes, FL, USA or Fubuki; Asahi Intecc Co Ltd., Aichi, Japan) was placed at C2 level portion of the ipsilateral VA. A microcatheter (Prowler Select Plus; Cordis Neurovascular) with an inner diameter of 0.021 inches was navigated gently across the dissecting aneurysm sac to a distal of the VA with using a 0.014 inches microwire (Traxcess; MicroVention or Agility; Cordis). And then microwire was removed and the first Enterprise stent was gently deployed from the proximal VA to the distal branch of the parent artery covering the entire VA dissecting aneurysm sac. The stent size was determined according to the operator’s judgment based on the diameter of the parent vessels and the length of the lesion. And then, the microcatheter was again advanced through the stent deployed lesion to overlay the second LVIS stent for modify blood flow diversion from the VADA. The second LVIS stent was deployed with a focus on maximizing overlap with the first stent, placing the dissection aneurysm sac at its center. After the stent deployment, we conducted a follow-up angiography to evaluate the parent and branch vessel integrity and ensure the stagnation of flow within the target lesion. Following this, the microwire and microcatheter was removed, and we achieved hemostasis at the puncture site with a closure device.
As shown in Table 1, the mean age of the 28 patients diagnosed with VADAs was 51.11±8.18 years; 18 patients (64.3%) were male and 10 (35.7%) were female. The mean follow-up period was 29.11±17.32 months. The most common symptoms were headache (75.0%), followed by facial palsy (3.6%), gait disturbance (7.1%), dizziness (7.1%), and tremors (3.6%). Further, 53.6% of patients had a history of hypertension, and 21.4% of patients had a history of dyslipidemia. No patient had a family history of diabetes mellitus, and of the 28 patients, eight (28.6%) had a history of smoking.
As shown in Table 2, all patients had unruptured VADAs located in the V4 (intracranial) segment of the VA. The mean aneurysm size was 4.99±1.28 mm in diameter and 10.60±3.35 mm in length. Four patients (14.3%) had PICA involvement. Double-overlapping stents were successfully deployed in all 28 patients, and the patency of the VA on follow-up imaging was maintained and no VA occlusion was observed. There were no cases of procedural complications, such as in-stent thrombosis, obstruction, or dissection rupture, and all outcomes were confirmed to have an mRS of 0–1 upon outpatient follow-up. After a follow-up DSA examination conducted 6 to 12 months later, Raymond grade was assessed in 28 patients, with 24 of them resulting grade 1 (completely healed), three patients with grade 2 (residual neck with focal stenosis or dilatation), and the remaining one patient with grade 3 (residual sac with dissecting flap). The patient with grade 3 has been managed conservatively, continuing dual antiplatelet therapy and is currently under outpatient observation without any reported symptoms or additional treatment. In addition, none of the VADAs ruptured during the follow-up period.
A 53-year-old patient with a history of hypertension and benign prostatic hyperplasia presented to the emergency room three days after the sudden onset of severe headache, dizziness, and gait disturbance. An MRI examination revealed intramural hematoma indicative of VA dissection and a wall enhancing lesion (Fig. 1A and B). CT angiography showed fusiform dilatation below the right distal VA and the origin of the PICA (Fig. 1C). Based on the imaging findings and clinical symptoms, the patient was diagnosed with acute VA dissection. Subsequent DSA confirmed the angioarchitecture of the dissection (Fig. 1D). The patient was admitted to the general ward and started on dual antiplatelet therapy. One week later, they underwent a general induction procedure and received a double-overlapping stent. Follow-up DSA examination 11 months later showed normalization of the previously stenotic and dilated lesion due to dissection (Fig. 2). The patient remained free of specific symptoms and was assessed with a mRS score of 0 at the last follow-up.
In this study, our objective was to assess the clinical and angiographic outcomes of double-overlapping stents, specifically employing LVIS within Enterprise stents, for the management of VADAs. For patients with VADAs who do not respond to conservative treatment and those experiencing persistent symptoms or showing progressive patterns on follow-up imaging, a more aggressive approach through endovascular treatment is necessary to reduce the risk of stroke and intracranial hemorrhage. Prior to the development of endovascular devices, VADA treatment was often performed through surgical reconstructive therapy or internal trapping, clipping or bypass of the VA. However, the use of destructive techniques can lead to high complication rates, involving brain stem infarction, hydrocephalus, brain swelling, and intracerebral hemorrhage, especially if the PICA is involved. In 1998, Lylyk et al. [21] and Sekhon et al. [24] first proposed SAC as a treatment for ruptured VAs. SAC is a method used for treating injured blood vessels through the diversion of blood flow using stents while accelerating aneurysm occlusion by packing coils into VADAs. SAC, due to its lower risk of complications, has become an alternative to existing destructive techniques. A meta-analysis revealed that there was no significant difference about mortality and morbidity between destructive therapy, the double-overlapping stents method, and SAC embolization [12]. However, in the case of SAC, instability may occur while placing the coil into the aneurysm sac, and there is a risk that PICA occlusion may occur if the PICA is the origin of the aneurysm [28].
As an alternative, treatment that only employs stents for deployment to damaged VADAs has emerged. Stent-only placement stabilizes the dissection-induced intimal flap and maintains blood flow in the injured blood vessel without clogging. It also promotes thrombosis by effecting a change in the blood flow to the VA, resulting in inflammation, which diverts blood flow away from the aneurysm sac. It also maintains the flow of the PICA branch, facilitating the patency of the VA. Previous reports on the treatment of VADAs have shown that single stent placement was insufficient for aneurysm occlusion, and double-overlapping stents placement achieved better results with respect to blood flow conversion and aneurysm occlusion [22,28]. This study is significant as we were able to demonstrate that reconstructive therapy using overlapping stents has proven stability and effectiveness compared with other VADA treatments.
Because porosity, cell architecture, and the type of stent vary depending on manufacturers, it is important to establish a strategy for selecting the type of stent for use in the double-overlapping stent technique. LVIS with Enterprise stent deployment is a combination that appropriately utilizes the advantages of the structural characteristics of stents, as previously revealed in previous studies [16,26]. The Enterprise (Codman Neurovascular) is laser-cut from Nitinol hypotubes and is a self-expanding closed-cell design microstent. Enterprise stents tend to be limited by outbound bulging and luminal curve flattening. The VA is generally linear compared to other intracranial arteries; therefore, it is less likely to cause problems with stent ovalization or kinking, making the Enterprise suitable for use as a stent that provides scaffolding for the unconstrained segments (aneurysm neck) of VADAs. LVIS stents have advantages over other stents in terms of porosity and pore density; thus, they are excellent in terms of the flow diversion effect by reducing wall shear stress and velocity [26]. However, the outward expansion of both ends of the aneurysm neck causes uneven metal coverage distribution. These problems can be overcome by minimizing outward expansion by deploying Enterprise stents.
The treatment outcomes for the total of ten cases that were excluded from the patient group were generally favorable. Among the ten cases, there were no procedural complications such as rupture or in-stent thrombosis. Specifically, upon detailed examination of follow-up angiography for the seven cases treated with the Enterprise within enterprise double-overlapping stent, results revealed Raymond grade 1 in three cases, grade 2 in three cases, and grade 3 in one case. Clinically, the follow-up mRS score was 2 for two individuals, while the remaining patients all demonstrated an mRS score of 1. Many patients primarily reported intermittent complaints of headache and dizziness following the procedure.
Among the two cases treated with Solitaire AB within Enterprise, one case showed Raymond grade 3 due to partial dissection resulting in blood flow stagnation, while the other patient had a favorable outcome with grade 1. For the individual treated with LVIS within LVIS double stent, follow-up DSA results indicated a favorable outcome with Raymond grade 1 and an mRS score of 1.
Table 3 shows a comprehensive literature review related to the overlapping stent-only technique for artery dissecting aneurysms. As shown in Table 3, there were differences in the selection of overlapping stent type. Kim et al. [14], Lv et al. [20], and Cho et al. [6] used the Enterprise with Enterprise stent for double-overlapping stents. We have chosen the combination of LVIS within the Enterprise stent as our optimal treatment strategy because a previous study has indicated that the combination of two Enterprise stents in a double-overlapping stent configuration has a drawback with a metal coverage rate of approximately 13%. This results in a reduced flow diversion effect compared to other combinations [15]. However, although not included in this study, we treated eight patients with a double-overlapping stent using a combination of two Enterprise stents during the study period. We believe that in cases where the fusiform dilatation due to dissection is not severe and there is not significant outbulging caused by dissection, this combination can still achieve a therapeutic effect through endothelialization. In the case of a double-overlapping stent combination using two LVIS stents, the metal coverage rate is approximately 36%, which is similar to that of single pipeline devices (PED; ev3/Covidien, Irvine, CA, USA). When considering the flow diversion effect alone, this combination can be a viable option [15]. However, as previously demonstrated in previous study and through our own experience, using two LVIS stents can lead to stent migration when passing the microcatheter to deploy the second stent, and they may not overlap in the desired position. Furthermore, the elevated metal coverage rate increases the risk of ischemic complications [19].
It is challenging to definitively assert the superiority of LVIS within Enterprise when considering the outcomes of different stent combinations. From the operator’s perspective, subjectively, deploying the LVIS double stent in the desired location proved challenging. In cases using the Enterprise within enterprise double-overlapping stent, the stiffness of the Enterprise stent limited its application to scenarios with less pronounced curvature of the blood vessel. If more cases are accumulated in the future, objective comparisons may become feasible. In conclusion, we believe that the double-overlapping stent with LVIS within the Enterprise stent is the optimal combination.
Recently, treatment using FDs has been approved for VADAs and has been widely performed. According to previous studies, treatment using overlapping stents is advantageous compared to FDs in terms of being able to produce a flow-diversion effect similar to FD and reducing the cost of the procedure [26]. In addition, FDs are characterized by reduced radial force; thus, they are not suitable if there is a narrowed vessel adjacent (pearl and string pattern) to VADAs. This suggests that overlapping stents may be more effective than FD [9]. While this limitation has recently been addressed, there are still many cases in which clinicians may not be familiar with the use of a FD for treatment, and there is a possibility of technical failure due to challenges in determining the appropriate size and length [13]. Considering these aspects, overlapping stents as a treatment for VADAs are considered sufficient. Double-stent placement without coiling may be considered less suitable than SAC for the immediate obliteration of VADAs, as a healing period for the injured VA needs to be considered. However, previous studies have revealed that if it is difficult to pack the coil in a fragile aneurysm sac, a favorable outcome can still be obtained with three or more multiple stents [28].
Our study had several limitations. This was a single-center retrospective study, a short follow-up period of 6 to 12 months and the comparative analysis was conducted using a small group of patients. All patients who underwent stent placement had unruptured VADAs. If the treatment results and prognosis-related factors were analyzed together in the cases of overlapping stents in patients with ruptured VADAs, we could compare those with the parent artery occlusion, patients’ clinical outcomes, and procedural complications. In the future, it is necessary to conduct studies on the outcomes of endovascular treatment strategies through a large-scale multicenter prospective study.
A double-overlapping stent, with a flow-diversion effect, is a safe and effective treatment for patients with VADAs. In particular, when using the LVIS stent within an Enterprise stent, it minimizes the bulging of the inner LVIS stent while maintaining flow diversion and stability. Therefore, both can be effectively utilized as overlapping stents.
Notes
Conflicts of interest
Yong Cheol Lim has been editorial board of JKNS since November 2017. He was not involved in the review process of this original article. No potential conflict of interest relevant to this article was reported.
References
1. Ahn JY, Han IB, Kim TG, Yoon PH, Lee YJ, Lee BH, et al. Endovascular treatment of intracranial vertebral artery dissections with stent placement or stent-assisted coiling. AJNR Am J Neuroradiol. 27:1514–1520. 2006.
2. Asai K, Nakamura H, Nishida T, Morris S, Sakaki T. Overlapping stent-assisted coil embolization for a ruptured intracranial vertebral artery dissection. J Surg Case Rep. 2017:rjx105. 2017.

3. Benndorf G, Herbon U, Sollmann WP, Campi A. Treatment of a ruptured dissecting vertebral artery aneurysm with double stent placement: case report. AJNR Am J Neuroradiol. 22:1844–1848. 2001.
4. Bhogal P, Brouwer PA, Söderqvist ÅK, Ohlsson M, Andersson T, Holmin S, et al. Patients with subarachnoid haemorrhage from vertebrobasilar dissection: treatment with stent-in-stent technique. Neuroradiology. 57:605–614. 2015.
5. Cerejo R, Bain M, Moore N, Hardman J, Bauer A, Hussain MS, et al. Flow diverter treatment of intracranial vertebral artery dissecting pseudoaneurysms. J Neurointerv Surg. 9:1064–1068. 2017.

6. Cho DY, Choi JH, Kim BS, Shin YS. Comparison of clinical and radiologic outcomes of diverse endovascular treatments in vertebral artery dissecting aneurysm involving the origin of PICA. World Neurosurg. 121:e22–e31. 2019.

7. Choi HH, Cho YD, Yoo DH, Kang HS, Han MH. LVIS-within-enterprise double-stent procedure without coiling beneficial as treatment of unruptured vertebral artery dissecting aneurysms. Interv Neuroradiol. 28:136–141. 2022.

8. Chung Y, Lee SH, Choi SK, Kim BJ, Lee KM, Kim EJ. Triple stent therapy for the treatment of vertebral dissecting aneurysms: efficacy and safety. World Neurosurg. 99:79–88. 2017.

9. Cohen JE, Gomori JM, Rajz G, Rosenthal G, El Hassan HA, Moscovici S, et al. Vertebral artery pseudoaneurysms secondary to blunt trauma: endovascular management by means of neurostents and flow diverters. J Clin Neurosci. 32:77–82. 2016.
10. Debette S, Compter A, Labeyrie MA, Uyttenboogaart M, Metso TM, Majersik JJ, et al. Epidemiology, pathophysiology, diagnosis, and management of intracranial artery dissection. Lancet Neurol. 14:640–654. 2015.

11. Guerrero WR, Ortega-Gutierrez S, Hayakawa M, Derdeyn CP, Rossen JD, Hasan D, et al. Endovascular treatment of ruptured vertebrobasilar dissecting aneurysms using flow diversion embolization devices: single-institution experience. World Neurosurg. 109:e164–e169. 2018.

12. Hernández-Durán S, Ogilvy CS. Clinical outcomes of patients with vertebral artery dissection treated endovascularly: a meta-analysis. Neurosurg Rev. 37:569–577. 2014.

13. Kim CH, Lee CH, Kim YH, Sung SK, Son DW, Lee SW, et al. Flow diverter devices for the treatment of unruptured vertebral artery dissecting aneurysm. J Korean Neurosurg Soc. 64:891–900. 2021.

14. Kim MJ, Chung J, Kim SL, Roh HG, Kwon BJ, Kim BS, et al. Stenting from the vertebral artery to the posterior inferior cerebellar artery. AJNR Am J Neuroradiol. 33:348–352. 2012.
15. Kim S, Yang H, Hong I, Oh JH, Kim YB. Computational study of hemodynamic changes induced by overlapping and compacting of stents and flow diverter in cerebral aneurysms. Front Neurol. 12:705841. 2021.

16. Lim YC, Shin YS, Chung J. Flow diversion via LVIS blue stent within enterprise stent in patients with vertebral artery dissecting aneurysm. World Neurosurg. 117:203–207. 2018.

17. Liu P, Li Z, Hu L, Liu Y, Li P, Zhu W, et al. Clinical characteristics, endovascular choices, and surgical outcomes of intracranial vertebral artery dissecting aneurysms: a consecutive series of 196 patients. J Neurosurg. 138:215–222. 2022.

18. Liu Q, Qi C, Zhang Y, Deng L, Li G, Su W. Low-profile visualized intraluminal support stent-only technique for intracranial aneurysms-a report of 12 cases with midterm follow-up. World Neurosurg. 129:e40–e47. 2019.

19. Liu XL, Wang B, Zhao LB, Jia ZY, Shi HB, Liu S. Overlapping stents-assisted coiling for vertebral artery dissecting aneurysm : LVIS stent within neuroform EZ stent. J Korean Neurosurg Soc. 65:523–530. 2022.
20. Lv N, Cao W, Larrabide I, Karmonik C, Zhu D, Liu J, et al. Hemodynamic changes caused by multiple stenting in vertebral artery fusiform aneurysms: a patient-specific computational fluid dynamics study. AJNR Am J Neuroradiol. 39:118–122. 2018.

21. Lylyk P, Ceratto R, Hurvitz D, Basso A. Treatment of a vertebral dissecting aneurysm with stents and coils: technical case report. Neurosurgery. 43:385–388. 1998.

22. Park SI, Kim BM, Kim DI, Shin YS, Suh SH, Chung EC, et al. Clinical and angiographic follow-up of stent-only therapy for acute intracranial vertebrobasilar dissecting aneurysms. AJNR Am J Neuroradiol. 30:1351–1356. 2009.

23. Schievink WI. Spontaneous dissection of the carotid and vertebral arteries. N Engl J Med. 344:898–906. 2001.

24. Sekhon LH, Morgan MK, Sorby W, Grinnell V. Combined endovascular stent implantation and endosaccular coil placement for the treatment of a wide-necked vertebral artery aneurysm: technical case report. Neurosurgery. 43:380–383. discussion 384. 1998.

25. Suzuki T, Hasegawa H, Ando K, Shibuya K, Takahashi H, Saito S, et al. Possibility of worsening flow diversion effect due to morphological changes of a stented artery with multiple overlapping stents for partially thrombosed vertebral artery aneurysms. Front Neurol. 11:611124. 2020.
26. Wang C, Tian Z, Liu J, Jing L, Paliwal N, Wang S, et al. Flow diverter effect of LVIS stent on cerebral aneurysm hemodynamics: a comparison with Enterprise stents and the Pipeline device. J Transl Med. 14:199. 2016.

Fig. 1.
High-resolution T2 MRI (A) and T1-enhanced MRI (B) images show the right unruptured vertebral artery dissecting aneurysm. Red arrow indicated intramural hematoma and wall-enhancing lesions. (C) Three-dimentional (3D) computed tomography angiography and (D) 3D rotational digital subtraction angiography images provide detailed views of vertebral dissecting aneurysm. MRI : magnetic resonance imaging.
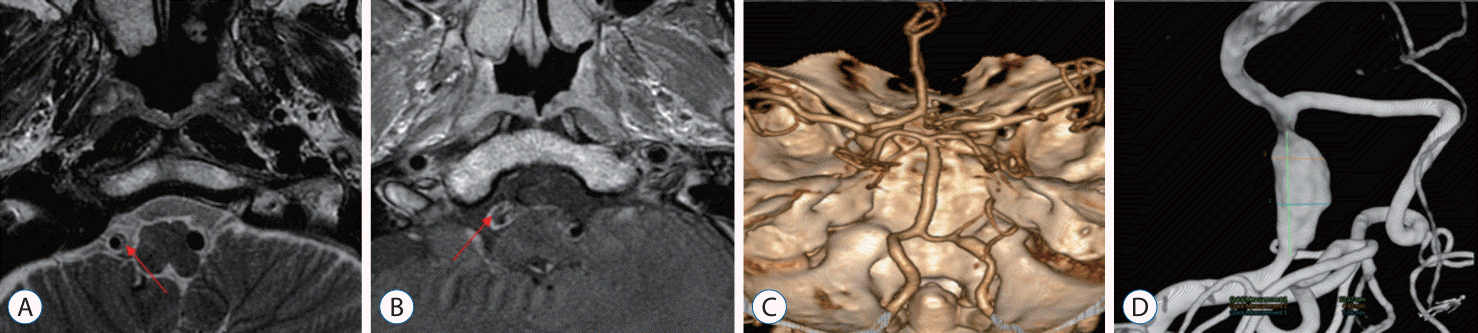
Fig. 2.
A : Right vertebral artery angiogram displaying the unruptured vertebral artery dissecting aneurysm (VADA). The VADA is located at the proximal site of the origin of the posterior inferior cerebellar artery with fusiform dilatation and stenosis. B : The VADA was successfully treated with deployment of one Enterprise (indicated by the black arrowheads) and one low-profile visualized intraluminal support stent (indicated by the white arrowheads) in a double-overlapping stent technique. C : Eleven-month follow-up angiogram revealed normalization and improved lesion stenosis after deployment of double-overlapping stent (indicate by the white and black arrowheads).
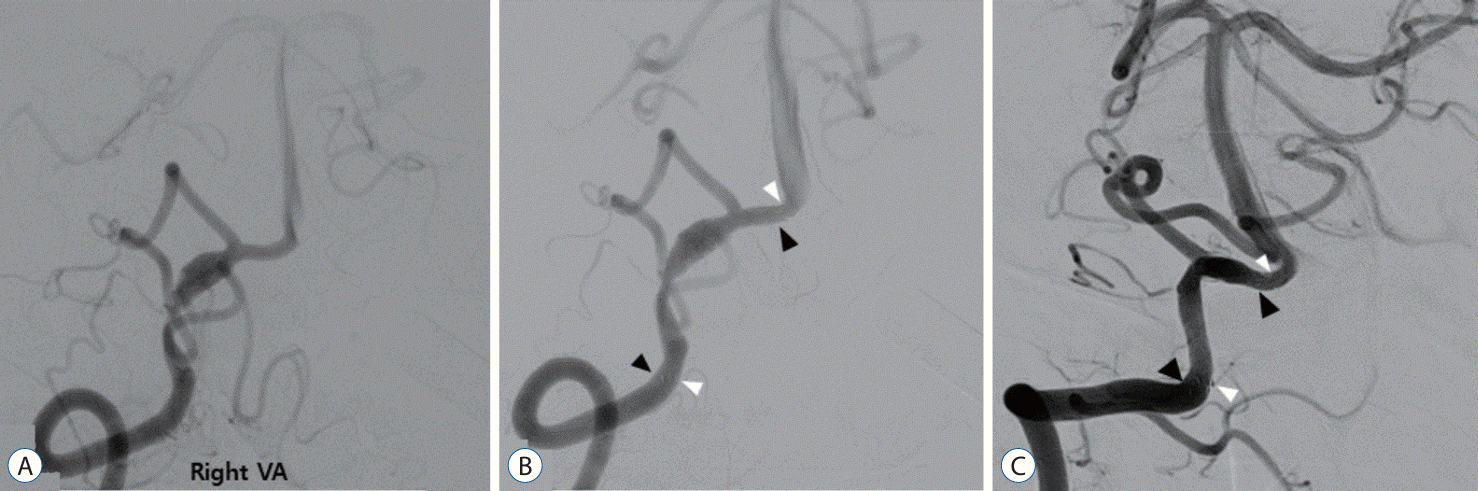
Table 1.
Demographic and clinical characteristics of 28 patients
Table 2.
Clinical characteristics and radiologic outcomes of 28 patients
Table 3.
comprehensive literature review related to the overlapping stent-only technique of vertebral artery dissecting aneurysms
| Study |
Number of patients |
Used stents | Outcome and complications | ||
|---|---|---|---|---|---|
| Total | Ruptured | Unruptured | |||
| Benndorf et al. [3] (2001) | 1 | 1 | S670 (Medtronic AVE) | Good outcome and no complication | |
| Ahn et al. [1] (2006) | 4 | 1 | 3 | S670, AVE Microdrive | Good outcome and no complication (mRS 0–1) |
| Park et al. [22] (2009) | 19 | 7 (1 triple) | 12 | Balloon-expandable coronary stents (Vision, FlexMaster, Driver, Zeta) | Good outcome and no complication (mRS 0–1) |
| Kim et al. [14] (2012) | 2 | 2 | Double Enterprise | Good outcome and no complication (mRS 0) | |
| Zhao et al. [28] (2013) | 25 | 25 (9 triple) | Neuroform, Leo, Enterprise | 19 favorable, 6 unfavorable (mRS 2–6), 2 procedural complication (vasospasm, ischemia) | |
| Bhogal et al. [4] (2015) | 9 | 9 (8 multiple) | Enterprise, Neuroform, LVIS | 7 favorable, 2 unfavorable (rebleeding, thrombosed) | |
| Chung et al. [8] (2017) | 8 | 1 | 7 (triple) | Enterprise, Solitaire, Neuroform | 7 good outcome, 1 recurrence (pseudoaneurysm) |
| Wang et al. [27] (2017) | 3 | 3 | LVIS, Enterprise | 2 good outcome, 1 unfavorable (occlusion) | |
| Lv et al. [20] (2018) | 5 | 5 | Double Enterprise | 3 good outcome, 2 unfavorable (thrombosis formation) | |
| Liu et al. [18] (2019) | 4 | 1 | 3 (1 triple) | Double LVIS | Good outcome and no complication |
| Cho et al. [6] (2019) | 4 | 1 (triple) | 3 | Double Enterprise | 3 good outcome, 1 unfavorable (parent artery occlusion with cerebellar infarct) |
| Suzuki et al. [25] (2020) | 4 | 4 | Double LVIS | 3 good outcome, 1 unfavorable (recanalization) | |
| Choi et al. [7] (2022) | 3 | 3 | LVIS within Enterprise | Good outcome and no complication (mRS 0–1) | |
| Liu et al. [17] (2022) | 48 | 4 | 44 | Not mentioned | N/A, 43.3% incomplete occlusion rate of aneurysm |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download