Abstract
Objective
Parkinson’s disease (PD) is one of the most prevalent neurodegenerative diseases, characterized by the loss of dopaminergic neurons in the substantia nigra pars compacta. The treatment of PD aims to alleviate motor symptoms by replacing the reduced endogenous dopamine. Currently, there are no disease-modifying agents for the treatment of PD. Zebrafish (Danio rerio) have emerged as an effective tool for new drug discovery and screening in the age of translational research. The neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) is known to cause a similar loss of dopaminergic neurons in the human midbrain, with corresponding Parkinsonian symptoms. L-type calcium channels (LTCCs) have been implicated in the generation of mitochondrial oxidative stress, which underlies the pathogenesis of PD. Therefore, we investigated the neuro-restorative effect of LTCC inhibition in an MPTP-induced zebrafish PD model and suggested a possible drug candidate that might modify the progression of PD.
Methods
All experiments were conducted using a line of transgenic zebrafish, Tg(dat:EGFP), in which green fluorescent protein (GFP) is expressed in dopaminergic neurons. The experimental groups were exposed to 500 μmol MPTP from 1 to 3 days post fertilization (dpf). The drug candidates : levodopa 1 mmol, nifedipine 10 μmol, nimodipine 3.5 μmol, diethylstilbestrol 0.3 μmol, luteolin 100 μmol, and calcitriol 0.25 μmol were exposed from 3 to 5 dpf. Locomotor activity was assessed by automated tracking and dopaminergic neurons were visualized in vivo by confocal microscopy.
Results
Levodopa, nimodipine, diethylstilbestrol, and calcitriol had significant positive effects on the restoration of motor behavior, which was damaged by MPTP. Nimodipine and calcitriol have significant positive effects on the restoration of dopaminergic neurons, which were reduced by MPTP. Through locomotor analysis and dopaminergic neuron quantification, we identified the neuro-restorative effects of nimodipine and calcitriol in zebrafish MPTP-induced PD model.
Conclusion
The present study identified the neuro-restorative effects of nimodipine and calcitriol in an MPTP-induced zebrafish model of PD. They restored dopaminergic neurons which were damaged due to the effects of MPTP and normalized the locomotor activity. LTCCs have potential pathological roles in neurodevelopmental and neurodegenerative disorders. Zebrafish are highly amenable to high-throughput drug screening and might, therefore, be a useful tool to work towards the identification of diseasemodifying treatment for PD. Further studies including zebrafish genetic models to elucidate the mechanism of action of the diseasemodifying candidate by investigating Ca2+ influx and mitochondrial function in dopaminergic neurons, are needed to reveal the pathogenesis of PD and develop disease-modifying treatments for PD.
Parkinson’s disease (PD) is one of the most prevalent neurodegenerative diseases, with loss of dopaminergic neurons in the substantia nigra pars compacta and accumulation of Lewy bodies composed of the protein α-synuclein [18,43]. Dopamine deficiency causes motor symptoms such as resting tremor, rigidity, bradykinesia, postural instability, and gait disturbance. Environmental and genetic factors are known to contribute to the etiology and disease progression of PD [40]. However, the exact mechanism of pathophysiology has not been elucidated and is still under study. The treatment of PD aims to alleviate motor symptoms by replacing reduced endogenous dopamine with levodopa and other dopaminergic modulators. Currently, there are no disease-modifying agents for the treatment of PD. Zebrafish (Danio rerio) have emerged as an effective tool for new drug discovery and screening in the age of translational research. Zebrafish are ideal for large-scale in vivo assays owing to their small size and rapid reproduction. In addition, the biggest advantage is that the drug can be administered to zebrafish, especially in the larval stage in a state where it can be dissolved in an aqueous environment, and the effect and toxicity can be determined [24]. The monoaminergic systems of the drug can be determined. The dopaminergic systems are also similar, with the difference being the absence of dopaminergic neurons in zebrafish mesencephalon [31]. The ventral diencephalon has been suggested to be the equivalent structure to substantia nigra, where dopamine transporter (DAT) and tyrosine hydroxylase are detected [15,34]. The neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) is known as one of the chemical-induced models for PD that causes a similar loss of dopaminergic neurons in the ventral diencephalon of larval zebrafish, which leads to corresponding Parkinsonian symptoms [2,22]. The possible mechanism of the selective loss of dopaminergic neurons in PD involves mitochondrial Ca2+ overload through L-type Ca2+ channel (LTCC), which leads to mitochondria dysfunction and production of reactive oxygen species (ROS), eventually resulting in apoptosis [13,23,38]. Thus, selective inhibition of LTCC as a way of rescuing neuronal cell death is emerging as a disease-modifying treatment in other neurodegenerative disorders of the central nervous system as well as PD [19,30,48]. In the present study, we investigated the neuro-restorative effect of LTCC inhibition on the MPTP-induced zebrafish PD model and suggested a possible candidate drug that might modify progression of PD.
The present study was approved by the Institutional Animal Care and Use Committee (IACUC) (KOREA-2022-0083) of Korea University College of Medicine and Institutional Biosafety Committee (IBC) (KUIBC-2022-0037).
All experiments were conducted by using a line of transgenic zebrafish, Tg(dat:EGFP), in which the green fluorescent protein (GFP) is expressed in the dopaminergic neurons (ventral diencephalon, amacrine cells in the retina, olfactory bulb, pretectum, and caudal hypothalamus) [46]. Adult zebrafish and embryos were maintained at 28°C in a recirculating aquaculture system and kept on a 14-hour light and 10-hour dark cycle. The larvae used in the experiment were obtained from an outcross between hetero-type adult dat:EGFP transgenic fishes. GFP-positive larvae were sorted using a fluorescence microscope (SMZ18; Nikon, Tokyo, Japan) 2 days post fertilization (dpf) after dechorionation.
MPTP (Sigma-Aldrich, Oakville, Canada) was dissolved in distilled water to 10 mmol as a stock solution and was diluted to make 100 µmol, 500 µmol, and 1 mmol as a working solution with E3 embryo medium (5 mmol NaCl, 0.17 mmol KCl, 0.33 mmol CaCl2, 0.33 mmol MgSO4) for behavior analysis and with 0.003% phenylthiourea to prevent pigmentation (1-phenyl 2-thiourea [PTU]; Sigma-Aldrich) for confocal microscopy. The control group was maintained at E3 or PTU without MPTP. The experimental groups were exposed to MPTP from 1 to 3 dpf, according to our study protocol (Fig. 1). Immunohistochemistry was performed to confirm the loss of dopaminergic neurons in the ventral diencephalon in Tg(dat:EGFP) zebrafish and to determine the concentration of MPTP used in the experiment. Larvae at 3 dpf were fixed in 4% paraformaldehyde and kept at 4°C overnight. Following fixation, larvae were then embedded in 1.5% agarose and 5% sucrose, rapidly frozen in isobutane and cooled in liquid nitrogen. The tissue was cryosectioned using Leica CM1850 (Leica Microsystems, Wetzlar, Germany) and mounted on glass slides. Tissue sections were washed three times for 5, 10, and 15 minutes in phosphate-buffered saline (PBS) and blocked in a blocking solution (bovine serum albumin [100 mg/mL] 500 µL and sheep serum [60 mg/mL] 500 µL mixed in PBS up to 20 mL) for 1–2 hours at room temperature. Sections were incubated with rabbit anti-tyrosine hydroxylase primary antibody at a 1 : 500 dilution overnight at 4°C and incubated with goat anti-mouse AlexaFluor 568 secondary antibody at a 1 : 1000 dilution. Images were analyzed using a confocal microscope (C5U-X1; Nikon). A concentration of 1 mM was found to cause the most severe reduction in dopaminergic neurons in the ventral diencephalon at 3 dpf (Fig. 2A). Compared to the control group, the number of dopaminergic neurons decreased in proportion to the MPTP concentration, and it was revealed that the number of dopaminergic neurons decreased by 50% in the MPTP 500 µM treatment group (Fig. 2A). The 500 µM MPTP concentration was chosen after the preliminary study.
The candidate drugs were exposed from 3 to 5 dpf, according to the study protocol (Fig. 1), to identify the neuro-restorative effect following MPTP treatment. The candidate drugs in the present study were known to inhibit LTCC in the previous study, except levodopa, which is an available pharmacotreatment for PD addressing symptomatology. The group was not exposed to any drug candidate after MPTP treatment and was administered diluted dimethylsulfoxide (DMSO). Nifedipine is a calcium channel blocker (CCB) of the dihydropyridine class that blocks LTCC. It is used as a drug for angina pectoris, hypertension, Raynaud’s phenomenon, and premature birth [27,30]. Nimodipine is an LTCC antagonist known to pass through the human brain-blood barrier, originally developed as a treatment for hypertension, but currently prevents vasospasm due to subarachnoid hemorrhage [21,37]. Diethylstilbestrol (DES) is a nonsteroidal estrogen drug used as hormonal therapy for estrogen deficiency in menopausal women, breast cancer treatment, and treatment of prostate cancer in men. Studies have shown that estrogen inhibits LTCC and has antioxidant effects [26,39,44]. Calcitriol, 1,25-dihydroxycholecalciferol, the active form of vitamin D, mainly increases the absorption of calcium in the intestine, thereby increasing the concentration of calcium in the blood. In a mouse experiment, a study on LTCC inhibition in the pre-frontal lobe was reported [11,14,29]. Luteolin is a yellow dye compound extracted from the plant with flavone, a flavonoid. Luteolin has been reported to inhibit the LTCC of the smooth muscle of the intestine [42,49]. To determine the optimal concentration of the drugs, zebrafish were exposed to the concentration of the drug used in the previous literature, and the maximum concentration of the drugs that did not induce any morphological changes was selected; levodopa 1 mmol, nifedipine 10 µmol, nimodipine 3.5 µmol, DES 0.3 µmol, luteolin 100 µmol, and calcitriol 0.25 µmol (Fig. 3).
Larval behavior analysis was performed 5 dpf after drug exposure. Swimming performances were automatically detected and tracked for 20 minutes using a digital video tracking system including a camera connected to a computer system using EthoVision 3.1 software (Noldus information Technology, Wageningen, Netherlands). Behavioral analysis was conducted on morphologically normal larvae. Larvae were raised in E3 for behavioral analysis and arrayed in the wells of a 48-well plate. After acclimation for 5 minutes, swimming performance was recorded and all digital tracks were analyzed for total distance covered in each well under light conditions [31,35]. Data were analyzed by averaging the distance moved per larvae during each 20 minutes. Each test was independently repeated twice.
Immunohistochemistry described in the 2.2 MPTP treatment was performed to identify dopaminergic neurons in the Tg(dat:EGFP) line and to confirm degradation of dopaminergic neurons according to the concentration of MPTP (Fig. 2A). For the quantification of dopaminergic neurons following MPTP and drug treatment, taking advantage of the transgenic line, in vivo confocal imaging was performed by immobilizing the whole larva ventrally (Fig. 2B), and the efficiency of imaging was increased by cutting off the head of the larvae and immobilizing several heads at once (Fig. 2C). After larvae were anesthetized (tricaine, 0.01%), heads of larvae were mounted in glycerol and imaged using a Nikon C5U-X1 confocal microscope at ×20 magnification with an argon laser (C488nm) to excite GFP, as a stack of optical sections were taken approximately 2 µm apart in the z-dimension. Ten fish/ group were used and experiment were repeated two times. The maximum intensity projection images used for the study were produced using the NIS-Elements software (Nikon, Mississauga, Canada). The number of dopaminergic neurons (GFP-positive neurons) present in the stack with the maximum intensity projection imaging was calculated [45]. The results are expressed as a percentage of the dopaminergic neurons present in the control group [50,51].
All statistical analyses were performed using the GraphPad Prism 7 software (GraphPad, San Diego, CA, USA). The comparisons of differences among control, MPTP + DMSO, and MPTP + drugs were performed using the Kruskal-Wallis Rank Sum Test, followed by Mann-Whitney test. Statistical graphs are expressed as the mean±standard error of the mean.
Forty-eight fish/group were used, and the experiment was repeated twice. The change in the velocity of each group over 20 minutes is shown in Fig. 4. A 500 µmol concentration of MPTP (DMSO group) produced a significant decrease in swimming speed (0.1525±0.4275 mm/s) (Table 1) compared to the control group (0.8643±1.0561 mm/s). Levodopa (0.3859±1.0647 mm/s, p<0.033), nimodipine (0.3817±0.7040 mm/s, p<0.001), DES (0.4834±0.9761 mm/s, p<0.001), and calcitriol (0.4889±1.2085 mm/s, p<0.001) significantly attenuated behavioral deficits induced by 500 µmol MPTP at 5 dpf (Table 1). In the present study, levodopa, nimodipine, DES, and calcitriol were shown to have significant positive effects on the restoration of locomotor behavior caused by MPTP.
Ten fish/group were used, and the experiments were repeated twice. The in vivo confocal microscopy images of each group are shown in Fig. 5. Exposure of 1 dpf zebrafish larvae to 500 µmol for 48 hours resulted in 46% reduction in dopaminergic neurons in the ventral diencephalon (Fig. 6) compared to the control group. To investigate whether the drugs exerted a rescue effect against MPTP-induced dopaminergic neuron loss, 1 dpf zebrafish larvae were treated with MPTP for 48 hours which was then replaced with drugs for another 2 days. As shown in Fig. 6, nimodipine (78.8%±2.6% of the control, p<0.001) and calcitriol (81.8%±2.9% of the control, p<0.001) significantly restored dopaminergic neurons from injury caused by pre MPTP treatment. Levodopa and DES did not show significant restorative effects against MPTP-induced dopaminergic neuron loss in zebrafish larvae. The restorative effects of each drug in dopaminergic neurons and locomotor behavior are summarized in Table 2.
This study demonstrated that the effect of MPTP on locomotor behavior and dopaminergic neurons in zebrafish larvae was restored by nimodipine and calcitriol using Tg(dat:EGFP). Administration of levodopa and DES could rescue the locomotor deficit, but not the loss of dopaminergic neurons. The current study is significant in that it investigated disease-modifying treatments for PD using a zebrafish drug screening system, since no agent has been shown to attenuate disease progression in PD. Development of disease-modifying agents from pharmacotherapies that address symptomatology is necessary and critical.
It is now well recognized that MPTP-induced Parkinsonism serves as a model for PD in mammals including mice to primates, and recently zebrafish [2,12,33]. In glial cells, MPTP is converted to 1-methyl-4-phenylpyridinium (MPP+). The DAT carries MPP+ into dopaminergic neurons following its release from the glia [25]. By interfering with respiratory enzymes and generating oxidative damage, MPP+ is thought to trigger cell death of dopaminergic neurons when it accumulates in the mitochondria [28]. Previous studies investigated the MPTP sensitivity of zebrafish in embryos, larvae, and adults. They identified that in response to MPTP, adult zebrafish displayed behavioral alterations, including decreased locomotor activity. Dopaminergic neurons in zebrafish embryos and larvae were notably impacted by MPTP as well as behavior alterations [2,22]. Since we used larvae model in the present study, we identified both the decrease of dopaminergic neurons and locomotor behavior in swimming response in response to MPTP.
L-type calcium channels (LTCCs) have emerged as drug targets in central nervous system disorders [5,16,19,20,30,41,48]. There are two classes of LTCCs; LTCCs with CaV1.2 pore-forming subunit which are found in the majority of LTCCs and LTCCs with CaV1.3 pore-forming subunit which are known to be associated with the production of mitochondrial oxidant stress, which underlies the pathogenesis of PD [19]. This oxidative stress may impair mitochondrial function and increase mitophagy, making cells more susceptible to other proteostatic stressors, and eventually contributing to cell death [41]. Recent research on the pathogenesis of PD proposed that selective vulnerability of dopaminergic neurons is caused by these neurons’ inherent capacity for spontaneous pacemaking, which depends on LTCCs with the CaV1.3 pore-forming subunit, resulting in increased calcium entry into the cells [4,32]. Inhibition of these channel reduces the need for Ca2+ and the susceptibility of these neurons to toxins, suggesting potential neuroprotection and neuro-restoration [5]. Selectively antagonizing these channels might be a potential disease-modifying treatment for PD, but there should not be any complication following blocking of LTCCs.
In the present study, nimodipine (3.5 µM) had a positive effect on neurogenesis following MPTP treatment with respect to locomotor behavior and dopaminergic neurons. Nimodipine is a lipophilic CCB of the dihydropyridine class, known to pass through the human blood-brain-barrier (BBB), originally developed as a treatment for hypertension, but currently prevents cerebral ischemia following vasospasm due to subarachnoid hemorrhage [3,7]. Previous studies suggested neuroprotective effects of nimodipine in MPTP-induced neurotoxicity in non-human primates [21,37]. Kupsch et al. [21] reported that nimodipine pretreatment almost completely prevented (in a dose-dependent manner) the MPTP-induced reduction in nigral tyrosine hydroxylase immunoreactive cells, but it did not alleviate the behavioral deficits in MPTP-treated monkeys or counteract striatal neurotoxin-induced dopamine depletion. These results point to different mechanisms of action for MPTP-induced neurotoxicity at the nigral versus the striatal level, suggesting that nimodipine protects against MPTP-induced neurotoxicity at the cellular nigral level but not at the synaptic striatal level [21]. Another study by Singh et al. [37] reported that nimodipine dose-dependently reduced the parkinsonian symptoms and loss of swimming ability in mice caused by MPTP. It enhanced mitochondrial oxygen consumption, decreased the generation of ROS in the striatal mitochondria, and arrested MPTP-induced loss of dopaminergic tyrosine hydroxylase-positive neurons in the substantia nigra [37]. These studies, which vary from the current study in that they found nimodipine to have neuroprotective effects, are significant because they provided evidence that nimodipine, an LTCC blocker, prevented the MPTP-induced loss of dopaminergic neurons. As isradipine, a dihydropyridine CCB, has been shown to be neuroprotective in animal models and has undergone a phase 3 clinical trial (although it failed to show efficacy) [1], further studies are necessary to confirm the neurorestorative effect of nimodipine on PD. The most biologically active metabolite derived from the secosteroid hormone vitamin D is calcitriol (1,25-dihydroxyvitamin D3). In the current study, the administration of calcitriol rescued the locomotor deficit and loss of dopaminergic neurons in the MPTP-induced PD zebrafish model. Normal neurodevelopment is greatly influenced by signaling processes of the vitamin D receptor (VDR). It has been discovered that VDR signaling is crucial for a number of neurobiological processes throughout development, including axon growth, neural differentiation, neurotransmitter synthesis, and modulation of neurotrophic factors [8,9]. The importance of vitamin D in cognitive processes, derivatives of vitamin D metabolites and VDR proteins has been discovered in the brain, with the largest densities expressed in the substantia nigra and the hippocampus [6]. In animal studies, altered vitamin D levels or VDR activity may result in behavioral impairments that last into adulthood. It follows that neurobehavioral consequences from exposure to substances that interfere with VDR signaling during development would be predicted [8]. Oliveri et al. [29] reported that the majority of the compounds known to affect VDR function significantly altered the locomotor behavior of exposed zebrafish; however, the direction of the impact, that is, hypo- or hyperactivity, differed according to the compounds. Vitamin D is known to be involved in regeneration and neurodevelopment. Han et al. [14] identified that alfacalcidol and vitamin D analogs enhanced the regeneration of injured zebrafish hearts, while VDR blockade inhibited regeneration. While VDR blockage prevented regeneration, alfacalcidol promoted regeneration in wounded zebrafish hearts14). The neuro-restorative effect of calcitriol may be achieved by enhancing the regeneration of dopaminergic neurons injured by MPTP, applying the idea that vitamin D controls regeneration. In addition, mental illnesses such as schizophrenia have been related to disruption of the VDR signaling pathway and vitamin D deficiency throughout development [36]. Gooch et al. [11] investigated whether 1,25 (OH)2D modulated LTCCs in the developing prefrontal cortex by using calcium imaging, electrophysiology, and molecular biology. The authors concluded that suboptimal concentrations of 1,25 (OH)2D could alter brain maturation through modulation of LTCCs signaling and might be a risk factor for schizophrenia. VDR proteins are highly expressed in the substantia nigra; as previously mentioned, the relationship between vitamin D and LTCCs might be critical for neurodevelopment. Further studies confirming that vitamin D selectively affects LTCCs with the CaV1.3 pore-forming subunit are needed to draw conclusions [10].
Zebrafish can be a useful tool to work toward the identification of disease-modifying treatments for PD with high-throughput drug screening. Prior to rodent or mammalian models and clinical trials, zebrafish are useful for quickly identifying the efficacy and toxicity of drug candidates owing to their rapid reproduction and development. Furthermore, zebrafish larvae can easily be treated with various substances by waterborne exposure [10]. In the present study, we revealed the effect of drugs on the restoration of dopaminergic neurons using transgenic lines with GFP-labelled DA neurons, Tg(dat:EGFP). This line greatly aided in the in vivo imaging of neuro-restorative compounds but also enabled rapid screening of different drug concentrations and treatment regimes. The BBB is developed at 3 dpf and is structurally and functionally similar to that of higher vertebrates [17,43,47]. When MPTP is administered to zebrafish larvae from 1 to 3 dpf, when the BBB is not well established, it can spread rapidly throughout the brain and cause loss of dopaminergic neurons. When the candidate drugs were administered to zebrafish after 3 dpf, the mature BBB posed an obstacle to drugs entering the brain. Only drugs that cross the BBB can rescue MPTP-induced insults in the dopaminergic neurons. Nimodipine and calcitriol, which showed neuroprotective effects in the current study, can be considered effective because they crossed the BBB in zebrafish. Therefore, a possible disease-modifying compound for PD should cross the BBB. Based on our experience, we suggest that zebrafish not only offers high throughput screening of drug candidates but also in vivo imaging revealing the pathogenic mechanisms before subjecting drug to mammals testing, which can save times and number of mammals.
The present study has several limitations. First, we did not differentiate between the types of LTCCs in the current study. Further studies are needed to validate the mechanism of action of nimodipine and calcitriol regarding which LTCCs have been antagonized to have neuro-restorative effects, consequently changes in Ca2+ influx, and mitochondrial function in dopaminergic neurons. Second, we used a chemical MPTP-induced zebrafish model of PD. Although MPTP has been widely accepted for environmental toxins which may contribute to idiopathic PD, causing PD-like symptoms and selective degeneration of dopaminergic neurons in various animal model, few mutations are known to be associated with familial cases and especially alpha-synuclein, component of Lewy bodies has been suggested to play a critical role in pathogenesis of idiopathic PD. Further studies with genetic models and combined with genetic and chemical models are required to identify the pathogenesis and progression of PD and develop disease-modifying treatment for PD.
The present study identified the neuro-restorative effects of nimodipine and calcitriol in an MPTP-induced zebrafish model of PD. They restored dopaminergic neurons from the effects of MPTP and normalized the locomotor activity. LTCCs have potential pathological roles in neurodevelopmental and neurodegenerative disorders. Future clinical research appears to be promising to address whether nimodipine and calcitriol may be useful in the treatment of PD. Zebrafish are highly amenable to high-throughput drug screening and might, therefore, be a useful tool to work towards the identification of disease-modifying therapies for PD. In particular, in vivo imaging was facilitated using Tg(dat:EGFP) transgenic zebrafish with fluorescent dopaminergic neurons. Further studies including those on zebrafish genetic models to elucidate the mechanism of action of the disease-modifying candidate by investigating Ca2+ influx and mitochondrial function in dopaminergic neurons are needed to reveal the pathogenesis of PD and develop disease-modifying treatments for PD.
Notes
Conflicts of interest
Sang-Dae Kim has been editorial board of JKNS since May 2017. He was not involved in the review process of this original article. No potential conflict of interest relevant to this article was reported.
ACKNOWLEDGMENTS
This research was supported by the National Research Foundation of Korea (NRF), funded by the Ministry of Science and ICT (NRF-2021R1F1A1046947) and Korea University Ansan Hospital (Grant Number O2207231).
References
1. Biglan KM, Oakes D, Lang AE, Hauser RA, Hodgeman K, Greco B, et al. A novel design of a phase III trial of isradipine in early Parkinson disease (STEADY-PD III). Ann Clin Transl Neurol. 4:360–368. 2017.
2. Bretaud S, Lee S, Guo S. Sensitivity of zebrafish to environmental toxins implicated in Parkinson’s disease. Neurotoxicol Teratol. 26:857–864. 2004.

3. Carlson AP, Hänggi D, Macdonald RL, Shuttleworth CW. Nimodipine reappraised: an old drug with a future. Curr Neuropharmacol. 18:65–82. 2020.

4. Chan CS, Guzman JN, Ilijic E, Mercer JN, Rick C, Tkatch T, et al. ‘Rejuvenation’ protects neurons in mouse models of Parkinson’s disease. Nature. 447:1081–1086. 2007.

5. Chang CC, Cao S, Kang S, Kai L, Tian X, Pandey P, et al. Antagonism of 4-substituted 1,4-dihydropyridine-3,5-dicarboxylates toward voltagedependent L-type Ca2+ channels Ca V 1.3 and Ca V 1.2. Bioorg Med Chem. 18:3147–3158. 2010.

6. Cui X, Gooch H, Petty A, McGrath JJ, Eyles D. Vitamin D and the brain: genomic and non-genomic actions. Mol Cell Endocrinol. 453:131–143. 2017.

7. DiPalma JR. Nimodipine in subarachnoid hemorrhage. Am Fam Physician. 40:143–145. 1989.
8. Eyles DW, Burne TH, McGrath JJ. Vitamin D, effects on brain development, adult brain function and the links between low levels of vitamin D and neuropsychiatric disease. Front Neuroendocrinol. 34:47–64. 2013.

9. Eyles DW, Feron F, Cui X, Kesby JP, Harms LH, Ko P, et al. Developmental vitamin D deficiency causes abnormal brain development. Psychoneuroendocrinology 34 Suppl. 1:S247–S257. 2009.

10. Flinn L, Bretaud S, Lo C, Ingham PW, Bandmann O. Zebrafish as a new animal model for movement disorders. J Neurochem. 106:1991–1997. 2008.

11. Gooch H, Cui X, Anggono V, Trzaskowski M, Tan MC, Eyles DW, et al. 1,25-Dihydroxyvitamin D modulates L-type voltage-gated calcium channels in a subset of neurons in the developing mouse prefrontal cortex. Transl Psychiatry. 9:281. 2019.

12. Grünblatt E, Mandel S, Youdim MB. MPTP and 6-hydroxydopamineinduced neurodegeneration as models for Parkinson’s disease: neuroprotective strategies. J Neurol. 247 Suppl 2:II95–II102. 2000.
13. Guzman JN, Sanchez-Padilla J, Wokosin D, Kondapalli J, Ilijic E, Schumacker PT, et al. Oxidant stress evoked by pacemaking in dopaminergic neurons is attenuated by DJ-1. Nature. 468:696–700. 2010.

14. Han Y, Chen A, Umansky KB, Oonk KA, Choi WY, Dickson AL, et al. Vitamin D stimulates cardiomyocyte proliferation and controls organ size and regeneration in zebrafish. Dev Cell. 48:853–863.e5. 2019.

15. Holzschuh J, Ryu S, Aberger F, Driever W. Dopamine transporter expression distinguishes dopaminergic neurons from other catecholaminergic neurons in the developing zebrafish embryo. Mech Dev. 101:237–243. 2001.

16. Ilijic E, Guzman JN, Surmeier DJ. The L-type channel antagonist isradipine is neuroprotective in a mouse model of Parkinson’s disease. Neurobiol Dis. 43:364–371. 2011.
17. Jeong JY, Kwon HB, Ahn JC, Kang D, Kwon SH, Park JA, et al. Functional and developmental analysis of the blood-brain barrier in zebrafish. Brain Res Bull. 75:619–628. 2008.

19. Kang S, Cooper G, Dunne SF, Dusel B, Luan CH, Surmeier DJ, et al. CaV1.3-selective L-type calcium channel antagonists as potential new therapeutics for Parkinson’s disease. Nat Commun. 3:1146. 2012.

20. Kang S, Cooper G, Dunne SF, Luan CH, James Surmeier D, Silverman RB. Antagonism of L-type Ca2+ channels CaV1.3 and CaV1.2 by 1,4-dihydropyrimidines and 4H-pyrans as dihydropyridine mimics. Bioorg Med Chem. 21:4365–4373. 2013.

21. Kupsch A, Sautter J, Schwarz J, Riederer P, Gerlach M, Oertel WH. 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurotoxicity in non-human primates is antagonized by pretreatment with nimodipine at the nigral, but not at the striatal level. Brain Res. 741:185–196. 1996.

22. Lam CS, Korzh V, Strahle U. Zebrafish embryos are susceptible to the dopaminergic neurotoxin MPTP. Eur J Neurosci. 21:1758–1762. 2005.

23. Lee KS, Huh S, Lee S, Wu Z, Kim AK, Kang HY, et al. Altered ER-mitochondria contact impacts mitochondria calcium homeostasis and contributes to neurodegeneration in vivo in disease models. Proc Natl Acad Sci U S A. 115:E8844–E8853. 2018.

24. Lieschke GJ, Currie PD. Animal models of human disease: zebrafish swim into view. Nat Rev Genet. 8:353–367. 2007.

25. Miller GW, Gainetdinov RR, Levey AI, Caron MG. Dopamine transport ers and neuronal injury. Trends Pharmacol Sci. 20:424–429. 1999.
26. Nakajima T, Kitazawa T, Hamada E, Hazama H, Omata M, Kurachi Y. 17beta-estradiol inhibits the voltage-dependent L-type Ca2+ currents in aortic smooth muscle cells. Eur J Pharmacol. 294:625–635. 1995.

27. Nedergaard S, Flatman JA, Engberg I. Nifedipine- and omega-conotoxin-sensitive Ca2+ conductances in guinea-pig substantia nigra pars compacta neurones. J Physiol. 466:727–747. 1993.

28. Nicotra A, Parvez SH. Cell death induced by MPTP, a substrate for monoamine oxidase B. Toxicology. 153:157–166. 2000.

29. Oliveri AN, Glazer L, Mahapatra D, Kullman SW, Levin ED. Developmental exposure of zebrafish to vitamin D receptor acting drugs and environmental toxicants disrupts behavioral function. Neurotoxicol Teratol. 81:106902. 2020.

30. Ortner NJ, Striessnig J. L-type calcium channels as drug targets in CNS disorders. Channels (Austin). 10:7–13. 2016.

31. Panula P, Sallinen V, Sundvik M, Kolehmainen J, Torkko V, Tiittula A, et al. Modulatory neurotransmitter systems and behavior: towards zebrafish models of neurodegenerative diseases. Zebrafish. 3:235–247. 2006.

32. Parkinson Study Group. Phase II safety, tolerability, and dose selection study of isradipine as a potential disease-modifying intervention in early Parkinson’s disease (STEADY-PD). Mov Disord. 28:1823–1831. 2013.

33. Ramirez AD, Wong SK, Menniti FS. Pramipexole inhibits MPTP toxicity in mice by dopamine D3 receptor dependent and independent mechanisms. Eur J Pharmacol. 475:29–35. 2003.

34. Rink E, Wullimann MF. The teleostean (zebrafish) dopaminergic system ascending to the subpallium (striatum) is located in the basal diencephalon (posterior tuberculum). Brain Res. 889:316–330. 2001.

35. Sallinen V, Torkko V, Sundvik M, Reenilä I, Khrustalyov D, Kaslin J, et al. MPTP and MPP+ target specific aminergic cell populations in larval zebrafish. J Neurochem. 108:719–731. 2009.

36. Schoenrock SA, Tarantino LM. Developmental vitamin D deficiency and schizophrenia: the role of animal models. Genes Brain Behav. 15:45–61. 2016.
37. Singh A, Verma P, Balaji G, Samantaray S, Mohanakumar KP. Nimodipine, an L-type calcium channel blocker attenuates mitochondrial dysfunctions to protect against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinsonism in mice. Neurochem Int. 99:221–232. 2016.

38. Soman SK, Bazała M, Keatinge M, Bandmann O, Kuznicki J. Restriction of mitochondrial calcium overload by mcu inactivation renders a neuroprotective effect in zebrafish models of Parkinson’s disease. Biol Open. 8:bio044347. 2019.
39. Sugishita K, Li F, Su Z, Barry WH. Anti-oxidant effects of estrogen reduce [Ca2+]i during metabolic inhibition. J Mol Cell Cardiol. 35:331–336. 2003.

40. Sulzer D. Multiple hit hypotheses for dopamine neuron loss in Parkinson’s disease. Trends Neurosci. 30:244–250. 2007.

41. Surmeier DJ, Schumacker PT, Guzman JD, Ilijic E, Yang B, Zampese E. Calcium and Parkinson’s disease. Biochem Biophys Res Commun. 483:1013–1019. 2017.

42. Swaminathan A, Basu M, Bekri A, Drapeau P, Kundu TK. The dietary flavonoid, luteolin, negatively affects neuronal differentiation. Front Mol Neurosci. 12:41. 2019.

43. Vaz RL, Outeiro TF, Ferreira JJ. Zebrafish as an animal model for drug discovery in Parkinson’s disease and other movement disorders: a systematic review. Front Neurol. 9:347. 2018.

44. Wagner M, Moritz A, Volk T. Interaction of gonadal steroids and the glucocorticoid corticosterone in the regulation of the L-type Ca(2+) current in rat left ventricular cardiomyocytes. Acta Physiol (Oxf). 202:629–640. 2011.

45. Xi Y, Ryan J, Noble S, Yu M, Yilbas AE, Ekker M. Impaired dopaminergic neuron development and locomotor function in zebrafish with loss of pink1 function. Eur J Neurosci. 31:623–633. 2010.

46. Xi Y, Yu M, Godoy R, Hatch G, Poitras L, Ekker M. Transgenic zebrafish expressing green fluorescent protein in dopaminergic neurons of the ventral diencephalon. Dev Dyn. 240:2539–2547. 2011.

47. Xie J, Farage E, Sugimoto M, Anand-Apte B. A novel transgenic zebrafish model for blood-brain and blood-retinal barrier development. BMC Dev Biol. 10:76. 2010.

48. Yagami T, Ueda K, Sakaeda T, Itoh N, Sakaguchi G, Okamura N, et al. Protective effects of a selective L-type voltage-sensitive calcium channel blocker, S-312-d, on neuronal cell death. Biochem Pharmacol. 67:1153–1165. 2004.

49. Yang M, Zhou Y, Wan LL, Ye JZ, Lu HL, Huang X, et al. Luteolin suppresses colonic smooth muscle motility via inhibiting L-type calcium channel currents in mice. Gen Physiol Biophys. 39:49–58. 2020.

Fig. 1.
Schematic illustration of toxin and drug treatment for the neuro-restoration protocol. One day post fertilization (dpf), dechorionated zebrafish were treated for 48 hours with 500 μM 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). MPTP was replaced with candidate drugs and treatment was continued for another 48 hours. After treatment, in vivo imaging and behavioral analyses were performed to reveal the neuro-restorative effects of the drugs following MPTP exposure.
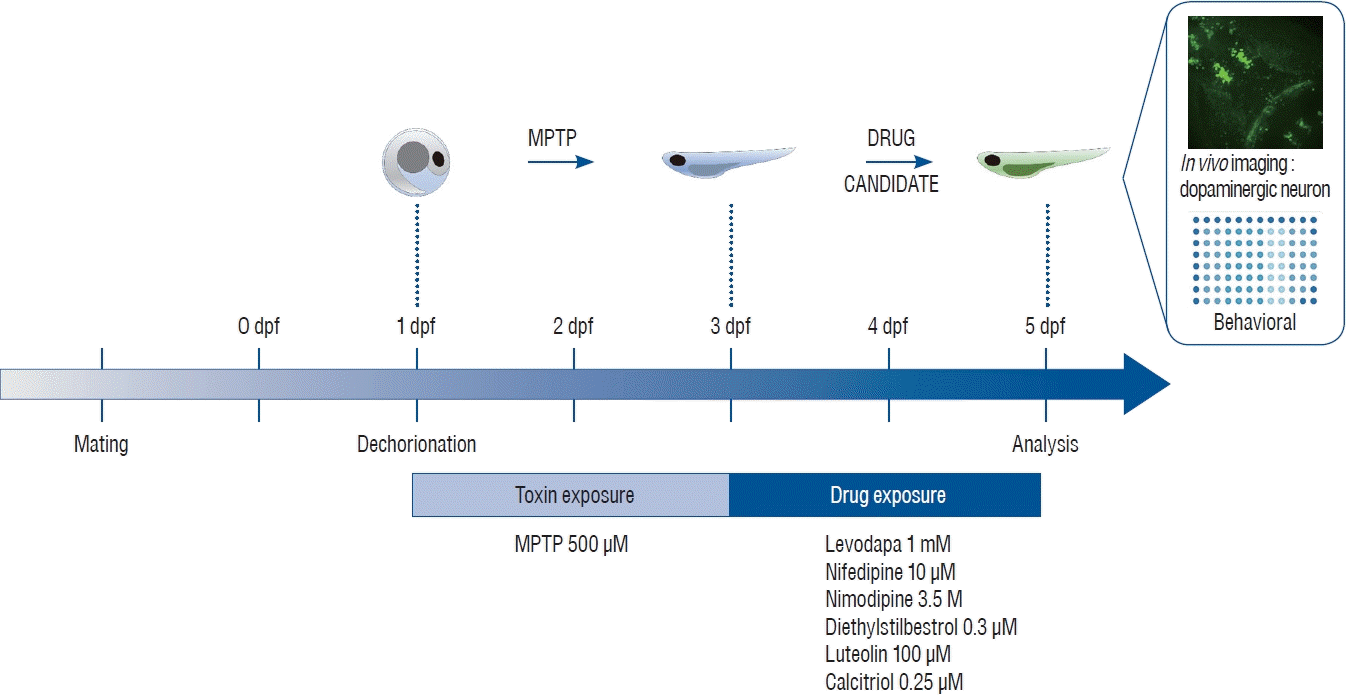
Fig. 2.
Zebrafish preparation for quantification of dopaminergic neurons. A : Immunostaining for tyrosine hydroxylase (TH) on horizontal cryosection of Tg(dat:EGFP) larvae at 3 days post-fertilization (dpf) following 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) treatment for 48 hours from 1 dpf with each MPTP concentration. B : In vivo imaging technique by fixing the whole larva ventrally. C : In vivo imaging technique by fixing the heads of larvae. Representative morphology of dopaminergic neurons in Tg(dat:EGFP) by in vivo imaging with each MPTP concentration. Ventral view, head at the left, tectum at the right.
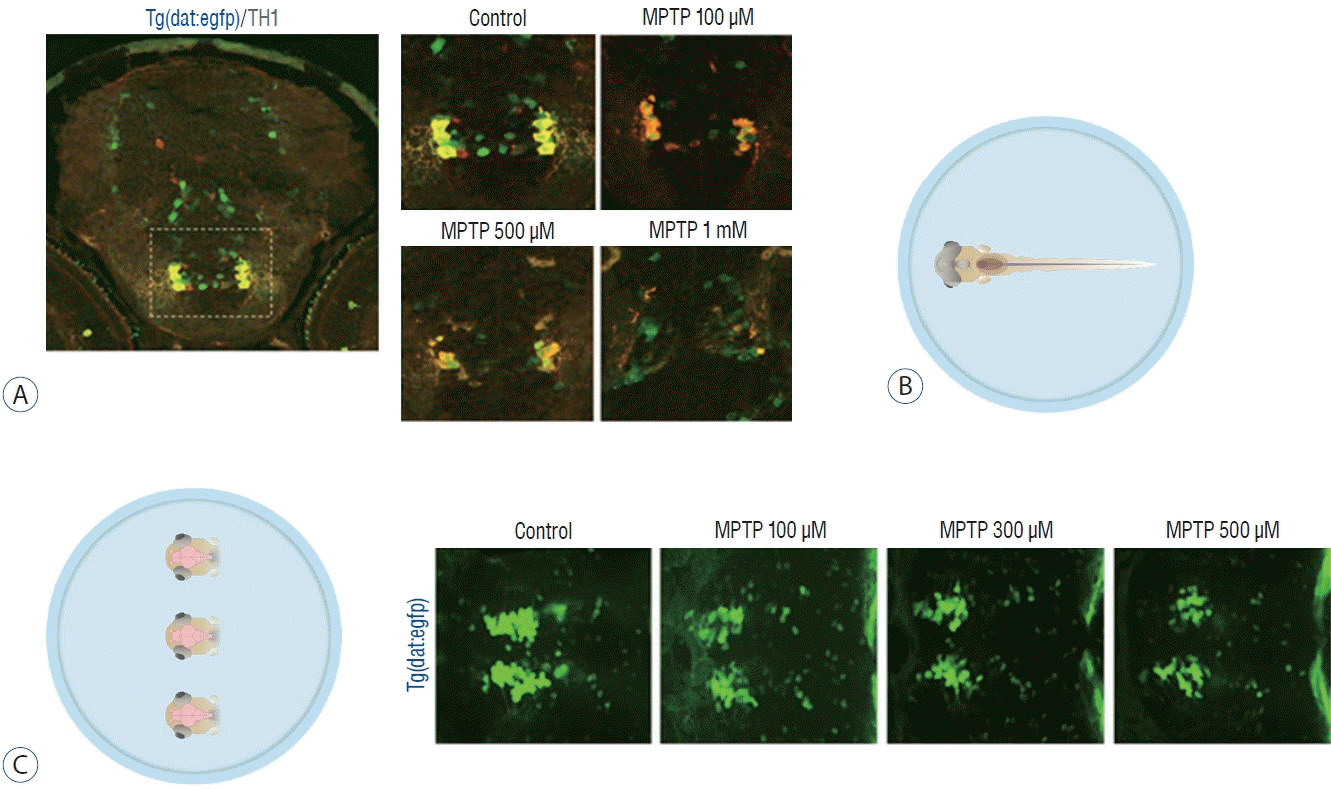
Fig. 3.
The maximum concentration of the drugs that did not induce any morphological changes. A : Levodapa 1 mmol. B : Nifedipine 10 μmol. C : Nimodipine 3.5 μmol. D : Diethylstilbestrol 0.3 μmol. E : Calcitriol 0.25 μmol. F : Luteolin 100 μmol.
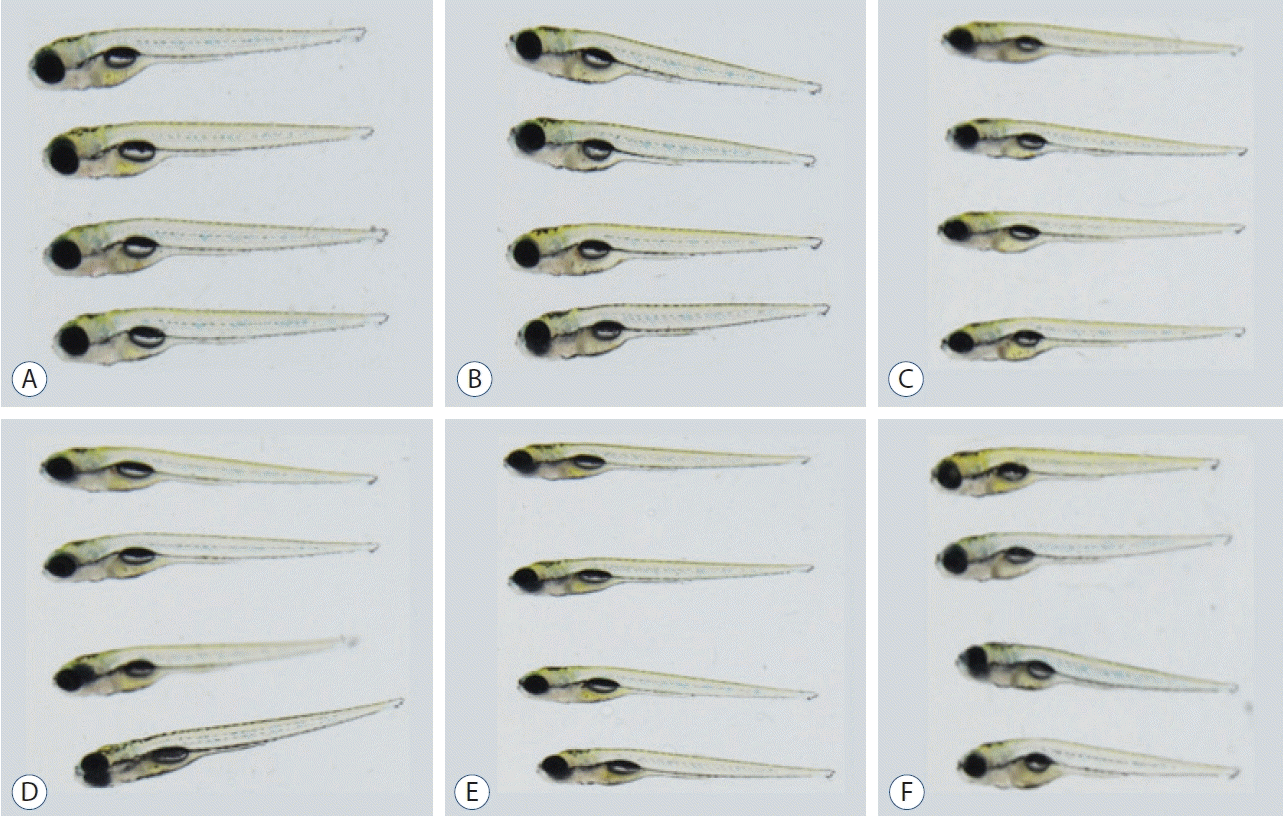
Fig. 4.
Locomotor behavior analysis for 20 minutes under light conditions. *p<0.05 vs. the MPTP followed by DMSO-treated group. E3 : embryo medium (5 mmol NaCl, 0.17 mmol KCl, 0.33 mmol CaCl2, 0.33 mmol MgSO4), DMSO : dimethylsulfoxide, DES : diethylstilbestrol, L-DOPA : levodopa, NIFE : nifedipine.
Fig. 5.
Representative morphology of dopaminergic neurons in Tg(dat:EGFP) in vivo imaging with each drug. Scale bars (gray line), 100 μm. Ventral view, head at the left, tectum at the right. DMSO : dimethylsulfoxide, DES : diethylstilbestrol.
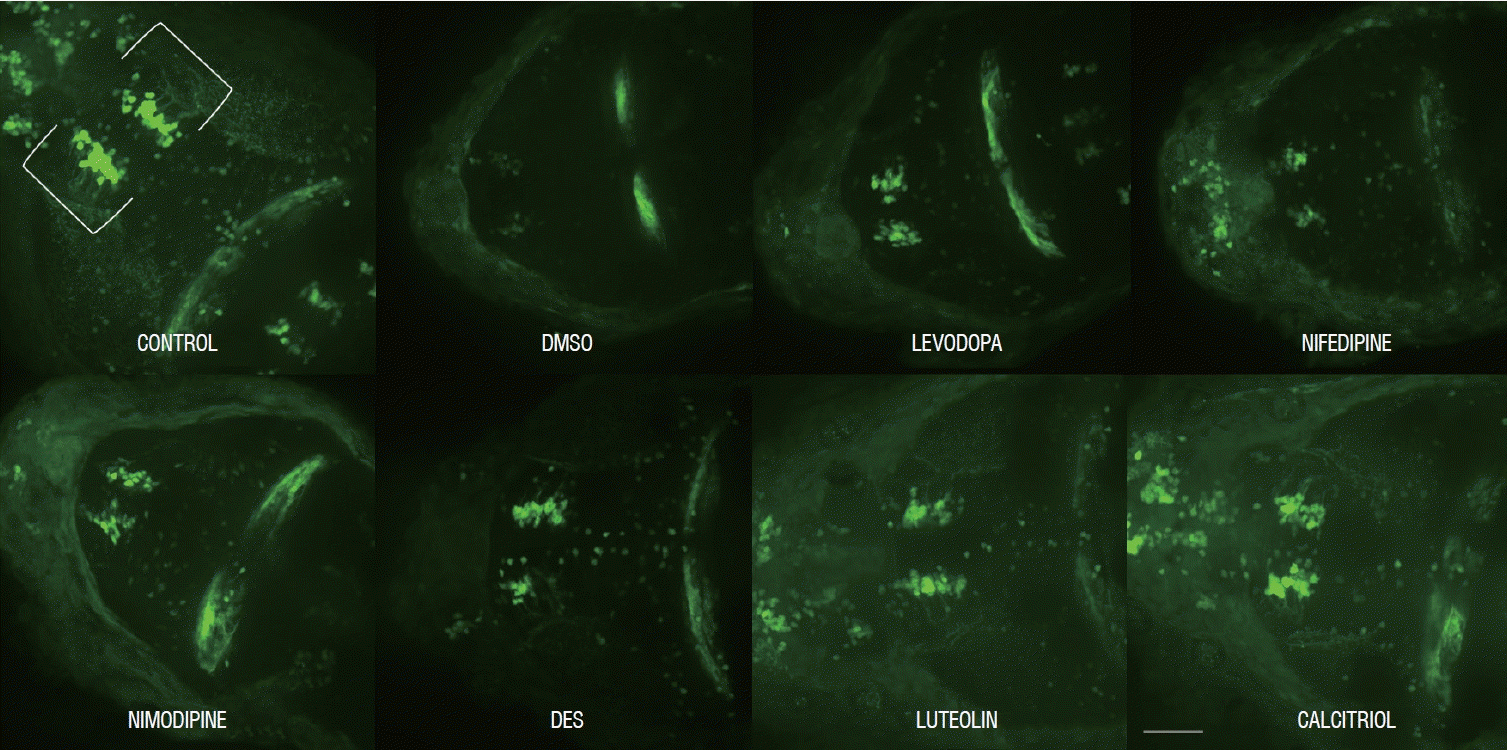
Fig. 6.
Quantitative analysis of dopaminergic neurons in Tg(dat:EGFP) zebrafish ventral diencephalon. Data are expressed as a percentage of the control group. Each bar represents mean±standard deviation. *p<0.05 vs. the MPTP followed by DMSO-treated group. DMSO : dimethylsulfoxide, DES : diethylstilbestrol.
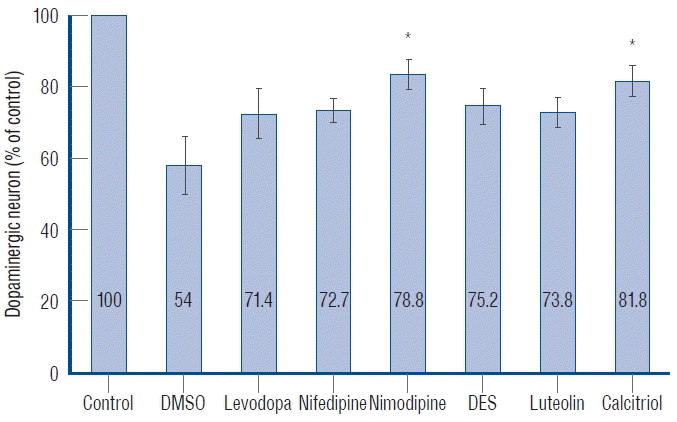
Table 1.
Locomotor behavior analysis for 20 minutes under light condition
| Group | Velocity (mm/sec) | p-value |
|---|---|---|
| Control | 0.8643±1.0561 | <0.001 |
| DMSO | 0.1525±0.4275 | - |
| Levodopa | 0.3859±1.0647 | 0.033* |
| Nifedipine | 0.3398±0.8773 | 0.532 |
| Nimodipine | 0.3817±0.7040 | <0.001* |
| DES | 0.4834±0.9761 | <0.001* |
| Luteolin | 0.3285±1.0148 | 0.449 |
| Calcitriol | 0.4889±1.2085 | <0.001* |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download