Abstract
Surgical treatment of refractory and extensive cerebral venous sinus thrombosis (CVST) has limited applications. Here, we describe an open, direct sinus thrombectomy in the early phase of extensive CVST. A 49-year-old man with extensive CVST that occurred after the coronavirus disease 2019 (COVID-19) vaccination and affected the drainage of the Labbé vein presented with clinical deterioration and left temporal hemorrhagic infarction. Since the patient had extensive CVST, we determined that systemic anticoagulation and endovascular treatment were not suitable treatment options. Therefore, we decided on an emergency surgical treatment and performed direct surgical thrombectomy. We followed extended suboccipital approach and made multiple incisions on the sinuses, exposing the posterior superior sagittal sinus to the transverse sigmoid junction. Consequently, the clinical condition of the patient dramatically improved, resulting in a favorable outcome with a modified Rankin scale score of 0. Performing emergency open surgical thrombectomy was a technically feasible treatment option that recanalize obstructed sinuses. Importantly, the patient recovered with a good clinical outcome. Early maximal surgical thrombectomy can be an effective and lifesaving method to treat extensive CVST with hemorrhagic infarction.
Cerebral venous sinus thrombosis (CVST) is a type of uncommon stroke that accounts for less than 1% of all strokes [19]. However, owing to the recent coronavirus disease 2019 (COVID-19) pandemic, incidences of CVST have increased with COVID-19 infection and vaccination-related CVST cases also being reported [7,18,20]. Despite various studies on and an increased interest in CVST in recent years, its management can be challenging due to the lack of a consistent treatment approach [17].
Unlike surgical advancements in treating other cerebrovascular diseases, the role of surgery in CVST is still limited [17]. Indeed, despite the wide use of anticoagulants and the development of an endovascular treatment, 3.4–15% of CVST patients die in the acute phase [1,2,6,10,17]. Thus, the role of early surgical intervention should not be overlooked in reducing mortality in cases of extensive and life-threatening CVST. To the best of our knowledge, this is the first report of a maximal extent of surgical thrombectomy with a conventional approach. Here, we report a case of extensive CVST after COVID-19 vaccination which was treated with direct surgical thrombectomy during the early stages of the disease.
A 49-year-old man visited the emergency department with progressive headaches, aphasia, and altered mentality and a Glasgow coma scale (GCS) score of 12. While he had no past medical history, he had received the mRNA-1273 COVID-19 vaccine (Moderna®; ModernaTx, Inc., Cambridge, MA, USA) 10 days prior to the onset of his symptoms. Laboratory test results, including blood count and clotting screening, were normal. However, computed tomography (CT) scan revealed a diffuse hemorrhage in his left temporal lobe (Fig. 1A). The CT angiography also revealed an extensive filling defect along the lower portion of the superior sagittal sinus (SSS), left transverse sinus, sigmoid sinus, and internal jugular vein (Fig. 1B-D).
Magnetic resonance imaging (MRI) and digital subtraction angiography (DSA) were performed immediately once the patient was admitted to the intensive care unit. The initial MRI of the brain demonstrated venous congestion and hemorrhagic infarction within the left temporal and occipital lobes (Fig. 1E and F). Furthermore, DSA confirmed impaired drainage of the Labbé vein owing to the pronounced thrombosis of the posterior SSS, left transverse sinuses, sigmoid sinuses, and internal jugular vein (Fig. 2A). Importantly, a key goal of this case was the reperfusion of the Labbé drainage, requiring the prompt removal of the thrombus. Therefore, we decided to proceed with an open emergency surgical treatment.
The patient was arranged in a three-quarter prone position using a Mayfield head clamp, a hockey stick incision was made, and the skin flap was reflected (Fig. 3A, B, and D). Posterior occipital muscles were then dissected from the occipital bone. Subsequently, suboccipital craniotomy was performed over the midline to expose all the affected sinuses, particularly the torcular sinus and the transverse-sigmoid junction. Following craniotomy, intraoperative indocyanine green (ICG) videoangiography was performed to confirm the exact location of thrombosis. Before any incisions were made, three or four anchoring sutures were placed (Fig. 3C and E). Two-centimeter longitudinal incisions were then made sequentially at four locations, including the SSS, transverse sinus, transversesigmoid junction, and torcula (Fig. 3B). We first performed the thrombectomy of the anterolateral portion of the transverse sinus, where the Labbé vein drained, and proceeded to perform the thrombectomy of the torcula last. For each incision, the thrombus was directly removed from the sinus using forceps and a 12 French Frazier suction (Fig. 3F). Thrombectomy was performed until each sinus was recanalized. Meanwhile, saline irrigation was also conducted during the procedure to prevent air embolisms. The sinus wall was then reconstructed using the existing anchoring sutures with a fibrinogen/thrombin-based collagen fleece (TachoSil®; Takeda Austria GmbH, Linz, Austria). Once the procedure was completed, patency and circulation of the venous sinuses were checked using ICG videoangiography.
Postoperative CT angiography and DSA revealed minor recanalization of the vein of Labbé, SSS, and transverse sinus (Figs. 2B, 4A, and B). On the fourth postoperative day, MRI showed reduced venous congestion (Fig. 4C). Notably, a follow-up DSA performed nine days after the surgery revealed that the obstructed sinuses, including Labbé, were fully recanalized (Fig. 2C). Additionally, on the second postoperative day, the patient’s consciousness level increased to a GCS score of 15 without neurologic deficits or complications. As an outpatient, he displayed no neurological deficits and had a modified Rankin scale score of 0 at the 6-month follow-up.
Anticoagulation treatment in CVST with intracranial hemorrhage (ICH) has been considered controversial [9], although systemic anticoagulation has been accepted as an initial treatment of CVST even in the presence of ICH [5,17]. Among CVST patients, the incidence of cerebral hemorrhage ranges from 35–39% and is usually associated with a poor outcome [16]. In addition, Boukobza et al. [3] found that a hemorrhage was more frequent in the temporal lobe when the vein of Labbé was blocked than that when the vein was not blocked. Moreover, Gazioglu et al. [8] reported lower complete recanalization rates in patients with extensive CVST. In our case, although Labbé drainage was blocked with extensive CVST and the presence of ICH, the patient recovered to a normal state without increased bleeding complications after surgery.
To date, few surgical techniques have been described for CVST, and these are summarized in Table 1 [4,11-15,21]. Although nine total cases have been published, the treatment concept in our case is different. In previous cases, thrombectomy was performed through one or two small incisions. Aspiration through a small feeding tube and infusion of tissue-type plasminogen activator (tPA) were the primary procedures. Furthermore, systemic anticoagulation was performed, and intraoperative tPA was administered in all nine published cases. However, in our case, we performed multiple 20 mm incisions and directly removed as many thrombi as possible using 12 French Frazier suction and forceps. Moreover, in previous cases, surgical thrombectomy was recognized as an auxiliary treatment modality for systemic anticoagulation, whereas in our case, we used open surgical treatment as the primary treatment modality.
Open surgical thrombectomy as a treatment modality for CVST was first reported in 1990 by Persson and Lilja [16]. They observed additional hemorrhages during the treatment period. Thus, they speculated that the basal ganglia, subcortical hemorrhage, and cerebellar hemorrhage that occurred postsurgery were caused by systemic anticoagulation drugs and streptokinase. In another report describing surgical thrombectomy using the Fogarty catheter and direct thrombolysis, new hemorrhages were observed after surgery wherein direct tPA was used; the authors attributed this to an Actilyse treatment [11]. In addition, epidural hemorrhage and subdural hemorrhage have been reported in other cases wherein thrombectomy with tPA infusion was performed [4,12].
As revealed in our initial work-up, an increase in infarction and hemorrhage owing to venous congestion was expected because of blocked Labbé drainage. Furthermore, since the total occlusion of the internal jugular vein and transverse sinus was extensive, it was difficult to expect spontaneous circulation recovery upon systemic anticoagulation. Retrograde venous catheterization for thrombolysis was also limited because of occlusion of the internal jugular vein. Moreover, venous congestion and hemorrhagic infarction resulting from an approximate totally impaired drainage of the Labbé were likely to increase, leading to deterioration in neurological functions, including speech function. Therefore, we decided to perform emergency surgical thrombectomy. To the best of our knowledge, this case presents a new treatment strategy that has not yet been demonstrated.
Presently, there is no established surgical treatment for CVST. In addition, since surgical intervention of the dural sinus is burdensome, various preoperative preparations need to be performed to prevent complications. In our presented case, we considered the prevention of air embolism during surgery to be crucial. Accordingly, the patient’s positioning was of primary importance and efforts were made not to position the head too high or too low at heart level. This is because when the head is too high, the possibility of air embolism increases, whereas when it is too low, an increase in venous congestion is expected. Second, it was important to determine the order of incisions. Thus, we first started performing the procedure from the far side of the right transverse sinus, where the Labbé vein was drained, and lastly performed a thrombectomy in the torcula. Consequently, blood continuously filled the inside of the sinus. Third, anchoring sutures were placed before the dura incision, as they enabled rapid and safe closure of dura when bleeding occurred. In addition, by lifting both ends of the thread, thrombectomy and saline irrigation inside the sinus could be effectively performed. Fourth, continuous irrigation of the sinus minimized the possibility of air embolism.
The present case shows that surgical treatment with direct thrombectomy in the early phase of extensive CVST may be a lifesaving procedure exhibiting favorable outcomes. This technique can be a feasible option in extensive or refractory CVST cases that were previously thought to be incurable.
Notes
References
1. Azin H, Ashjazadeh N. Cerebral venous sinus thrombosis--clinical features, predisposing and prognostic factors. Acta Neurol Taiwan. 17:82–87. 2008.
2. Boncoraglio G, Carriero MR, Chiapparini L, Ciceri E, Ciusani E, Erbetta A, et al. Hyperhomocysteinemia and other thrombophilic risk factors in 26 patients with cerebral venous thrombosis. Eur J Neurol. 11:405–409. 2004.
3. Boukobza M, Crassard I, Bousser MG, Chabriat H. Labbé vein thrombosis. Neuroradiology. 62:935–945. 2020.

4. Ekseth K, Boström S, Vegfors M. Reversibility of severe sagittal sinus thrombosis with open surgical thrombectomy combined with local infusion of tissue plasminogen activator: technical case report. Neurosurgery. 43:960–965. 1998.

5. Ferro JM, Bousser MG, Canhão P, Coutinho JM, Crassard I, Dentali F, et al. European Stroke Organization guideline for the diagnosis and treatment of cerebral venous thrombosis - endorsed by the European Academy of Neurology. Eur J Neurol. 2:1203–1213. 2017.

6. Ferro JM, Canhão P, Stam J, Bousser MG, Barinagarrementeria F; ISCVT Investigators. Prognosis of cerebral vein and dural sinus thrombosis: results of the International Study on Cerebral Vein and Dural Sinus Thrombosis (ISCVT). Stroke. 35:664–670. 2004.

7. Furie KL, Cushman M, Elkind MSV, Lyden PD, Saposnik G; American Heart Association/American Stroke Association Stroke Council Leadership. Diagnosis and management of cerebral venous sinus thrombosis with vaccine-induced immune thrombotic thrombocytopenia. Stroke. 52:2478–2482. 2021.

8. Gazioglu S, Eyuboglu I, Yildirim A, Aydin CO, Alioglu Z. Cerebral venous sinus thrombosis: clinical features, long-term outcome and recanalization. J Clin Neurosci. 45:248–251. 2017.

9. Guenther G, Arauz A. Cerebral venous thrombosis: a diagnostic and treatment update. Neurologia. 26:488–498. 2011.

10. Khealani BA, Wasay M, Saadah M, Sultana E, Mustafa S, Khan FS, et al. Cerebral venous thrombosis: a descriptive multicenter study of patients in Pakistan and Middle East. Stroke. 39:2707–2711. 2008.
11. Kourtopoulos H, Christie M, Rath B. Open thrombectomy combined with thrombolysis in massive intracranial sinus thrombosis. Acta Neurochir (Wien). 128:171–173. 1994.

12. Lechanoine F, Janot K, Herbreteau D, Maldonado IL, Velut S. Surgical thrombectomy combined with bilateral decompressive craniectomy in a life-threatening case of coma from cerebral venous sinus thrombosis: case report and literature review. World Neurosurg. 120:485–489. 2018.

13. Lee DJ, Ahmadpour A, Binyamin T, Dahlin BC, Shahlaie K, Waldau B. Management and outcome of spontaneous cerebral venous sinus thrombosis in a 5-year consecutive single-institution cohort. J Neurointerv Surg. 9:34–38. 2017.

14. Lee DJ, Latchaw RE, Dahlin BC, Dong PR, Verro P, Muizelaar JP, et al. Antegrade rheolytic thrombectomy and thrombolysis for superior sagittal sinus thrombosis using burr hole access. BMJ Case Rep. 2014; bcr2013011087. 2014.

15. Persson L, Lilja A. Extensive dural sinus thrombosis treated by surgical removal and local streptokinase infusion. Neurosurgery. 26:117–121. 1990.

16. Pongmoragot J, Saposnik G. Intracerebral hemorrhage from cerebral venous thrombosis. Curr Atheroscler Rep. 14:382–389. 2012.

17. Saposnik G, Barinagarrementeria F, Brown RD Jr, Bushnell CD, Cucchiara B, Cushman M, et al. Diagnosis and management of cerebral venous thrombosis: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 42:1158–1192. 2011.

18. See I, Su JR, Lale A, Woo EJ, Guh AY, Shimabukuro TT, et al. US case reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S vaccination, March 2 to April 21, 2021. JAMA. 325:2448–2456. 2021.

Fig. 1.
Preoperative imaging findings of sinus thrombosis, venous congestion, and hemorrhagic infarction. A : Initial non-contrast computed tomographic scan demonstrates left temporal cortical and subcortical hemorrhage (white arrow) and increased density of the left transverse and sigmoid sinus junctions due to a dense thrombus (black arrow). B : Computed tomographic venogram coronal view showing low density in the left internal jugular vein, representing sinus thrombosis (white arrow). C : Computed tomographic venogram axial view showing low density in the left internal jugular vein, representing sinus thrombosis (white arrow). D : Computed tomographic venogram coronal view showing low density in the superior sagittal sinus near the torcula and left transverse sinus representing sinus thrombosis (white arrows). E : Susceptibility-weighted magnetic resonance image showing hemorrhagic infarction in the left temporal lobe (black arrow) and venous congestion in the left temporal and occipital lobes (white arrows). F : Diffusion-weighted magnetic resonance image showing a high-intensity lesion in the left temporal lobe (white arrow).
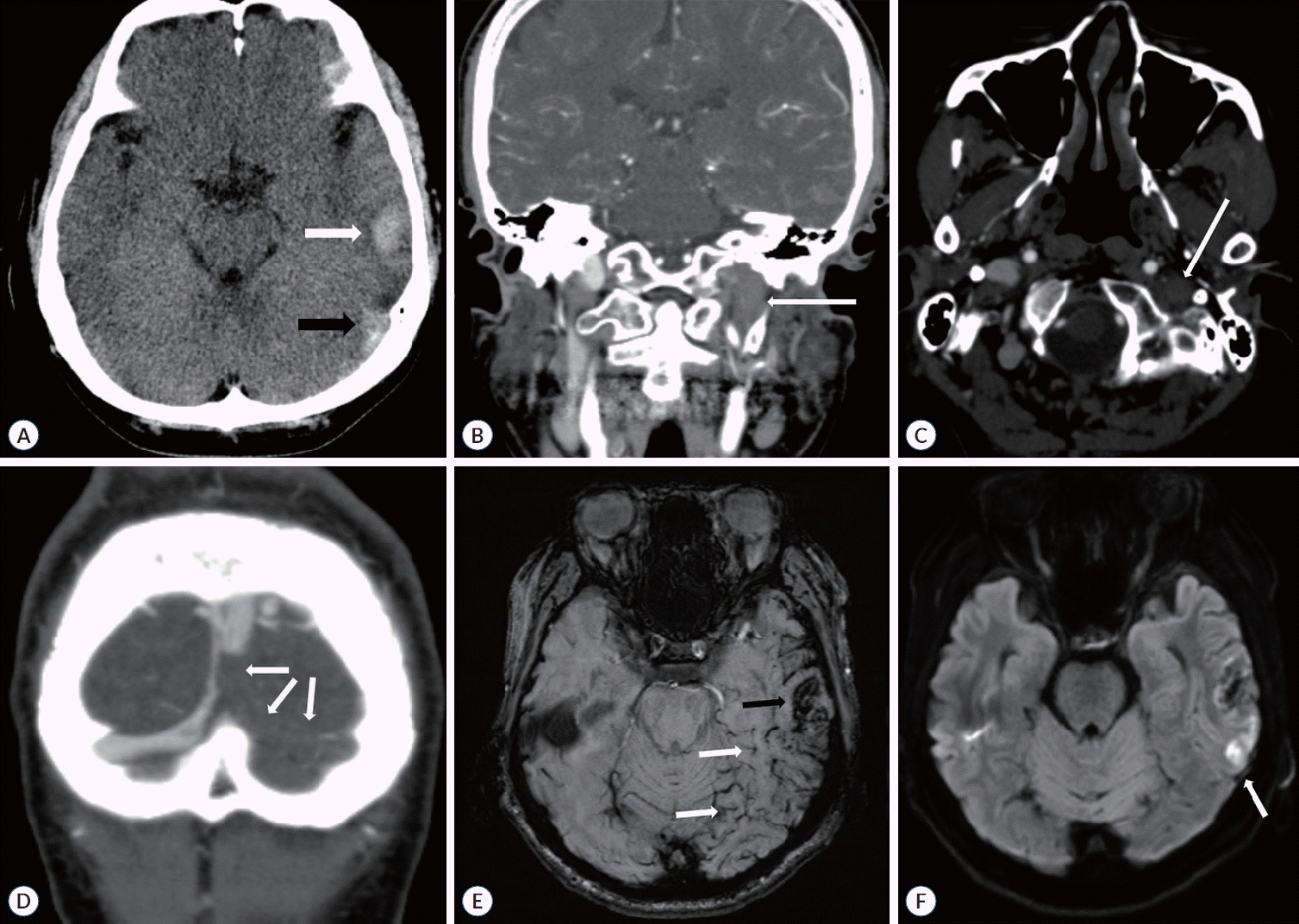
Fig. 2.
Digital subtraction angiography of left internal carotid artery venous phase in oblique view. A : Preoperative digital subtraction angiography demonstrates pronounced thrombosis of the superior sagittal sinus near the torcula (white arrows), left transverse sinus, and sigmoid sinus, preventing the drainage of the Labbé vein. B : Postoperative digital subtraction angiography demonstrates recanalization of the superior sagittal and transverse sinuses (white arrows) with patency restoration of the Labbé vein (black arrow). C : Nine days after operation, digital subtraction angiography demonstrates a more prominent restoration of patency in the superior sagittal sinus, transverse sinus, and Labbé vein (black arrow).
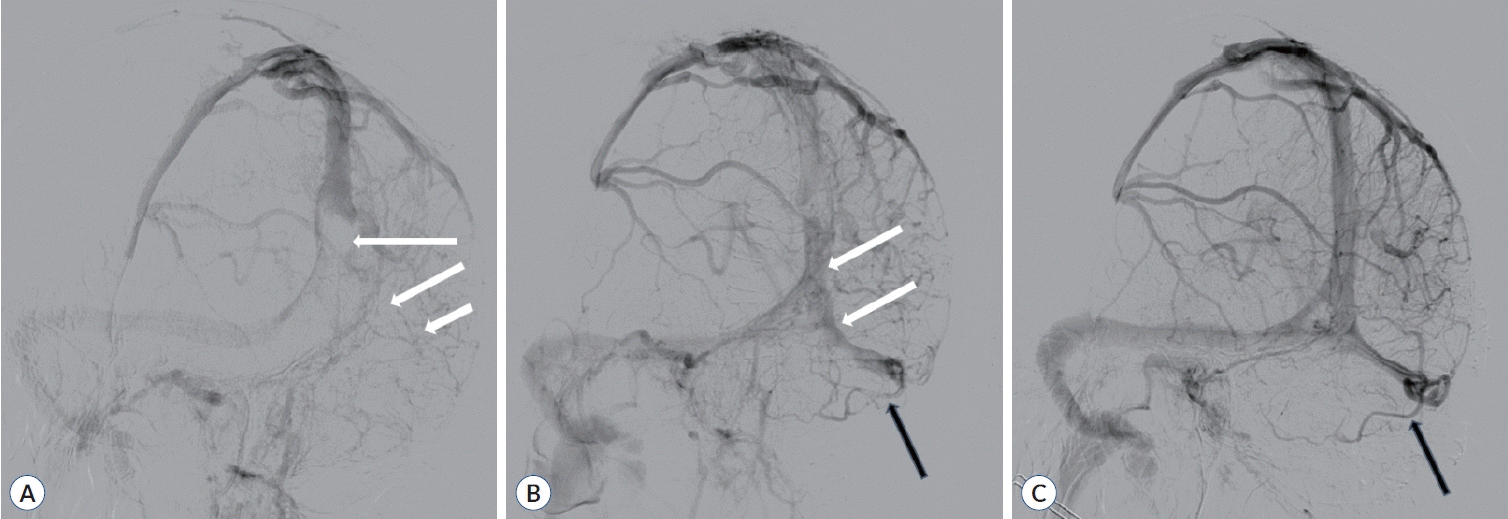
Fig. 3.
Surgical procedure. A : Schematic drawing of the operative position (Park-bench position). B : Schematic drawing indicating the locations of skin and dural incisions. C : Schematic drawing of the dural incision and anchoring sutures at the transverse sinus near the drainage site of Labbé. D : Operative image of the patient with a reflected skin flap after a hockey stick incision. The expected location of the transverse sinus was marked using forceps. E : Operative image of a dural incision with anchoring sutures at the transverse sinus near the drainage site of Labbé. F : Intraoperative findings during thrombectomy using Frazier suction and forceps. Continuous saline irrigation was applied to the torcula.
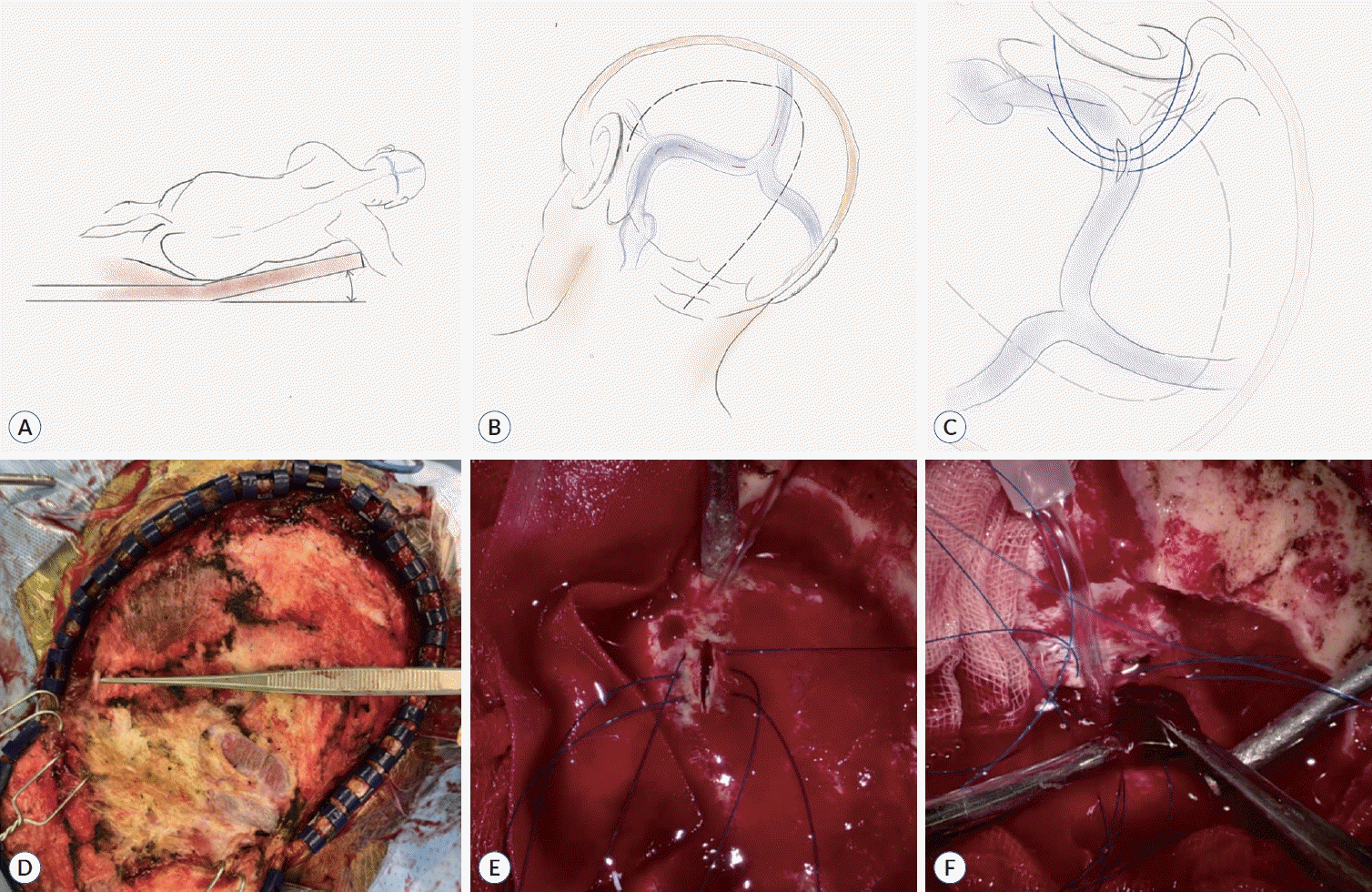
Fig. 4.
Postoperative imaging findings. A : Computed tomographic venogram coronal view showing recanalization of the superior sagittal and transverse sinuses (white arrows). B : Computed tomographic venogram sagittal view showing prominent restoration of patency in the Labbé vein (white arrow). C : Susceptibility-weighted magnetic resonance imaging showing no change in the amount of hemorrhagic infarction (black arrow) and decreased venous congestion in the left temporal and occipital lobes (white arrow).
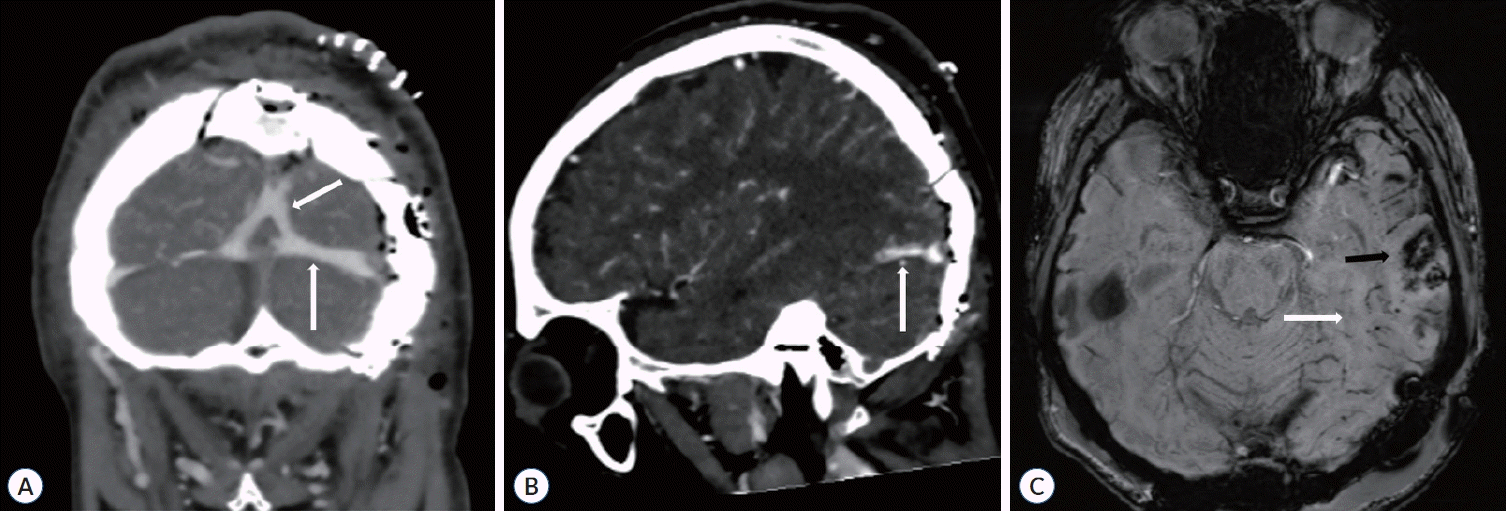
Table 1.
Reported cases of surgical thrombectomy with demographic information, technique for surgery, use of intraoperative tPA and systemic anticoagulation, initial and post-operative hemorrhage, and clinical outcome
| Study | Age (years) | Sex | Location of CVST | Surgical opproach | Thrombectomy method | Intraoperative tPA | Systemic anticoagulation | Initial hemorrahge | Post treatment hemorrhage | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| Persson and Lilja [15] (1990) | 27 | F | SSS, straight sinus, both transverse sinuses, vein of Galen | (1) craniotomy over sinus confluence | Forceps, feeding tube suction | Not used | Yes | X | Yes | Good |
| (2) craniotomy over bregma | Forceps, feeding tube suction | 24 hours via epidural catheter | ||||||||
| Kourtopoulos et al. [11] (1994) | 18 | F | SSS | Frontoparietal craniotomy | Fogarty catheter, infant feeding catheter | 5 mg tPA 4 hours and saline | Yes | Yes | Yes | Good |
| Ekseth et al. [4] (1998) | 27 | F | SSS | Craniotomy over vertex, 15–20 mm incision on sinus | Direct removal and baby feeding catheter aspiration | 8 mg tPA 4 hours and saline 24 hours | Yes | Yes | Yes (EDH) | Good |
| 19 | M | Partially occluded SSS | Yes | No | Good | |||||
| 24 | F | SSS, both transverse sinuses, straight sinus | Yes | No | Good | |||||
| Lee et al. [14] (2014) | 51 | F | SSS involving the torcula and right transverse sinus | Burr hole | Foley catheter aspiration, endovascular sheath | 9 mg tPA over 18 hours, three times | Yes | Yes | No | mRS score 2 |
| Lee et al. [13] (2017) | Fourth decade | NS | SSS, right transverse sinus, vein of Labbe, jugular vein | hemicraniectomy | SSS thrombectomy and thrombolysis | Thrombolysis done | Yes | Yes | NS | Death |
| Lechanoine et al. [12] (2018) | 45 | F | SSS, right transverse sinus | Bilateral craniectomy | Catheter insertion with small incision | 20 mg in situ thrombolysis (direct injection) | Yes | No | Yes (SDH) | Good |
| Inflatable balloon | ||||||||||
| Westwick et al. [21] (2019) | 15 | F | Anterior two-thirds of SSS | Rt craniectomy | Fogarty balloon catheter | 1 mg in situ thrombolysis (direct injection) | Yes | Yes | No | Good |
| Two midline burr hole | ||||||||||
| Present case | 49 | M | SSS near torcula, left transverse sinus, sigmoid sinus, internal jugular vein | Suboccipital craniotomy | Direct removal with suction and forceps | Not used | No | Yes | No | Good |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download