Abstract
Non-Hodgkin’s lymphoma (NHL) is the most common type of Gastrointestinal (GI) lymphoma with known complications such as bleeding, obstruction and perforation. In this article we present a 59-year-old male patient diagnosed with Peripheral T cell Lymphoma – Not Otherwise Specified (PTCL-NOS) with GI involvement was started on chemotherapy. On day 2 post completion of first cycle of chemotherapy, patient had presented to the emergency department with sudden onset abdominal pain and distension. On evaluation, he was diagnosed with multiple perforations in the small bowel. Patient underwent exploration with primary repair of few perforations and ileal resection with double barrel ileostomy. Chemotherapy plays an important role in the management of NHL. One well-known NHL consequence, intestinal perforation, can happen at the time of initial presentation or after starting chemotherapy. Surgeons should be aware of possibility of such complications and high-risk factors for perforation. At present, there is no role for elective surgery in GI lymphoma and is mainly reserved for complications like uncontrolled bleeding, obstruction or perforation.
Gastrointestinal (GI) tract is the most common extra-nodal site involved by lymphoma.1 Primary gastrointestinal tract lymphomas are uncommon; they typically affect the stomach but can affect any portion of the digestive system, including the esophagus and the rectum. Generally, because nodal illness is so extensive, secondary gastrointestinal involvement is somewhat common. Majority of GI lymphomas are of Non-Hodgkin type.2 Chemotherapy, radiation therapy and immunotherapy are among the treatment options for lymphomas with surgery primarily reserved for GI lymphoma complications such as bleeding, obstruction, perforation and fistula formation.3 A dangerous and sometimes fatal consequence, perforation can occur as the initial presentation in some cases or after chemotherapy is started in others.4 In this article, we present a case of Non Hodgkin’s Lymphoma (NHL) with gastrointestinal involvement that developed intestinal perforation very early after initiation of chemotherapy.
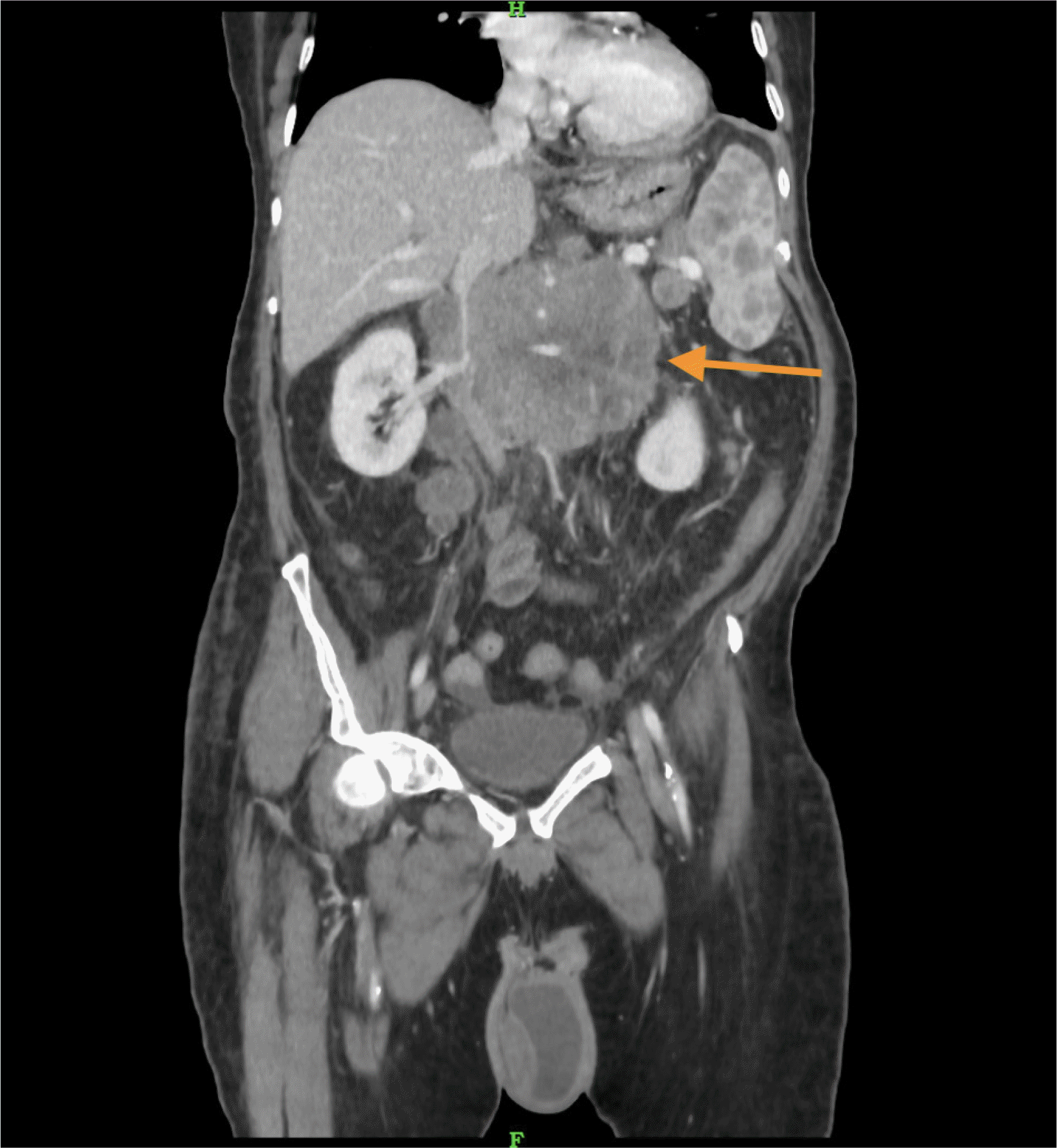
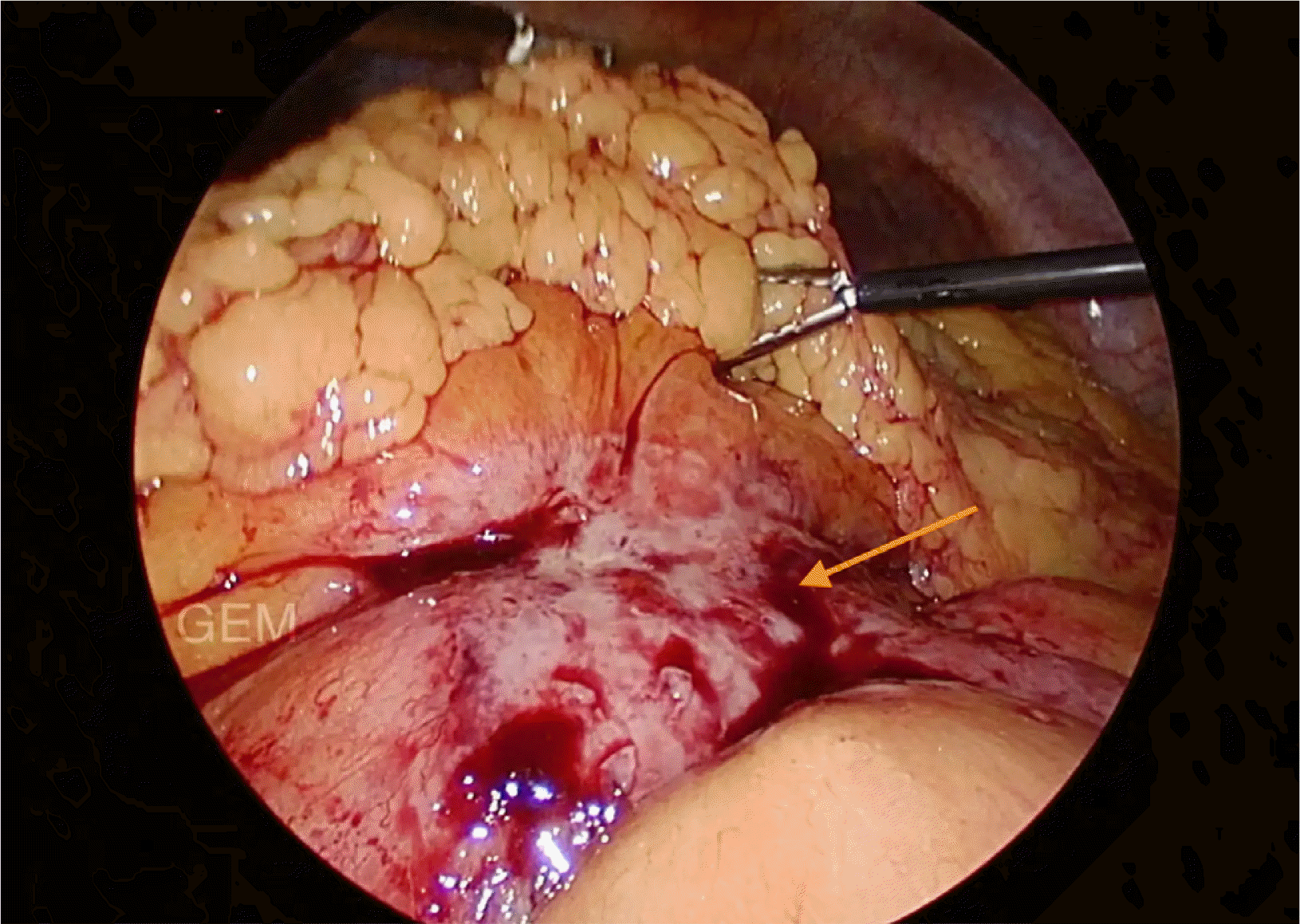
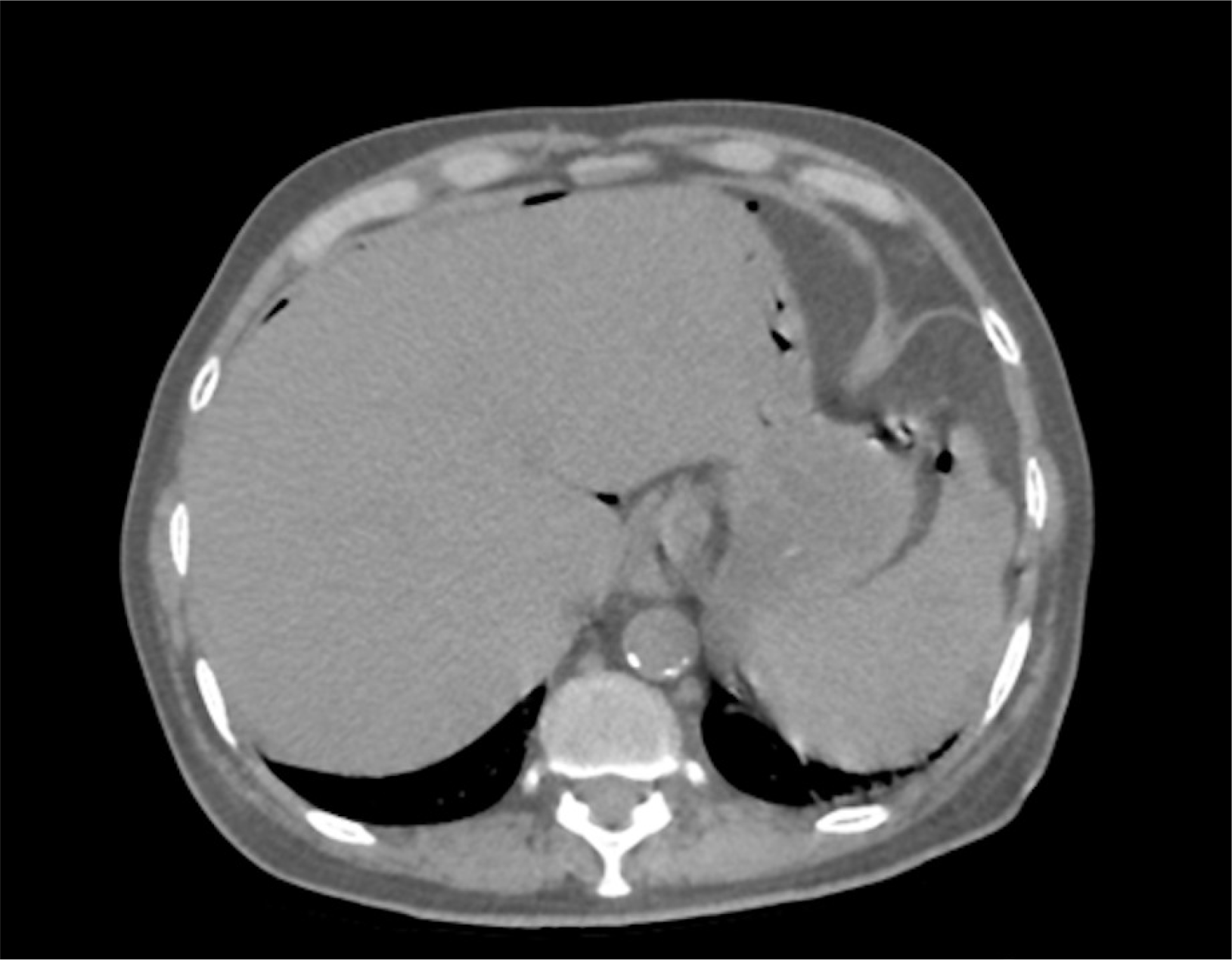
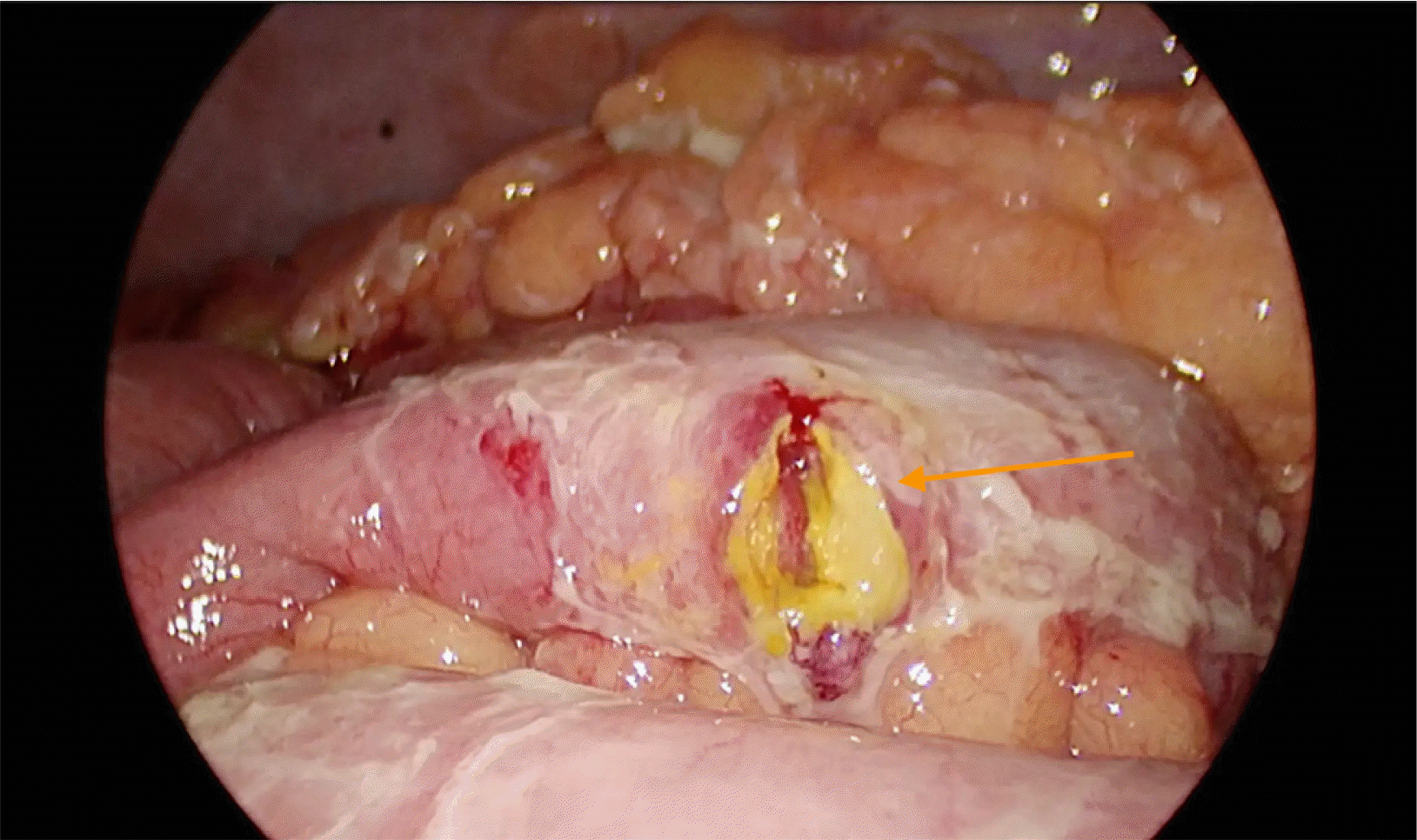
A 59-year-old male patient came with the complaints of mass per abdomen and diffuse abdominal pain for the past 3 months. He complained of fatigue and generalized weakness. He had significant loss of weight approximately 8 kilograms in the past 3 months and loss of appetite. No history of comorbidities or any previous surgery. Eastern Cooperative Oncology Group (ECOG) Performance status of 2. On abdominal examination a hard nodular mass of approximately 8×7 cm in size predominantly in the epigastric and umbilical region was palpable with mild diffuse tenderness. On evaluation contrast enhanced computed tomography (CECT) done showed heterogeneously enhancing retroperitoneal mass encasing aorta, inferior vena cava and bilateral renal vessels size approximately measures 10×8 cm. Also noted are multiple enlarged mesenteric lymph nodes visualized separately from the mass. Multiple hypodense hypo enhancing nodules noted in spleen (Fig. 1). Lactate dehydrogenase (LDH) levels were elevated - 352 U/L. Ultrasound guided trucut biopsy of the lymph nodal mass done was inconclusive. Following this, he underwent laparoscopic mesenteric lymph node biopsy (Fig. 2). Histopathology done showed lymph nodal tissue with atypical lymphoid proliferation consistent with NHL. Immunohistochemistry (IHC) was positive for CD3, CD4, CD8, CD2, CD7, CD68, Ki-67―50 to 55% and negative for CD20, CD30, CD56, PAX-5, PD-1, CD10, CD21, CD23, MUM-1 suggestive of Peripheral T cell Lymphoma―Not Otherwise Specified (PTCL-NOS). 18-Fluoro-deoxyglucose positron emission tomography (FDG PET) is preferred in T cell lymphoma done showed hypermetabolic conglomerate lymph nodal masses in the mesentery and retroperitoneum with findings consistent with CECT abdomen. Enlarged, discrete hypermetabolic left level I axillary, mediastinal, retrocrural, retroperitoneal, mesenteric, right common iliac, external iliac lymph nodes were present. Scattered hypermetabolic foci in the serosal aspect of small bowel jejunal loops were seen. Splenomegaly with multiple poorly enhancing hypermetabolic foci in the splenic parenchyma with features suggestive of lymphoproliferative disorder. The patient was diagnosed with of Non-Hodgkin's lymphoma, PTCL– NOS [Stage III A]. Based on International Prognostic Index (IPI) the patient is categorized as high-intermediate risk (3 points). With relapse free and overall survival rates at 5 years of about 50%. After multidisciplinary team discussion considering the general condition of the patient, although CHOP (Cyclophosphamide, doxorubicin, vincristine, prednisolone) is the preferred regimen, the patient was started on pre-phase chemotherapy – COP regimen (Cyclophosphamide, vincristine, prednisolone). The pre-phase chemotherapy consists of low dose chemotherapy prior to definitive chemotherapy, helps in increasing the performance status and has reduced toxic side effects in the initial phase of treatment. With cyclophosphamide 900 mg and vincristine 1 mg given as IV infusion on day 1, oral prednisolone 80 mg was given once daily for 4 days from day 2 to 5. Following initiation of the first cycle of chemotherapy patient had presented to the emergency department 2 days post the cycle with sudden onset abdominal pain and distension. He had history of fever for 1 day. On abdominal examination there was diffuse abdominal tenderness with guarding. Computed tomography (CT) abdomen done showed pneumoperitoneum with focal clumped air pockets adjacent to 2 separate areas in the proximal ileum suggestive of small bowel perforation (Fig. 3). Then the patient was taken up for emergency diagnostic laparoscopy. There was purulent free fluid in the peritoneal cavity with multiple perforations largest of size 2×1 cm (Fig. 4) noted in the proximal jejunum (100 cm distal to the duodenojejunal flexure) as well as few other sealed off perforations in a segment of terminal ileum with friable and dusky bowel segment forming a mass enclosed by omentum, 20 cm proximal to the ileocaecal junction. The main mass was located retroperitoneally and this mass was not in close relation to the site of small bowel perforation. Intervening bowel segment was edematous but with no evidence of friability or perforation. Since there were dense omental and inter-bowel adhesions, the procedure was converted to open and primary repair of the jejunal perforation with serosal jejunal patch placement, resection of the friable segment of ileum with double barrel ileostomy was done. The patient recovered well and was discharged on post operative day – 4 with a well-functioning stoma. Post operatively after 2 weeks patient has restarted chemotherapy.
According to the latest National Comprehensive Cancer Network (NCCN) guidelines, the first line therapy for patients with PTCL-NOS includes anthracycline based chemotherapy regimens - CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) or CHOP+etoposide (CHOEP) or dose-adjusted EPOCH (etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin). The use of brentuximab vedotin with CHP (cyclophosphamide, doxorubicin and prednisone) was superior to CHOP in patients with previously untreated CD-30 positive PTCL. First line consolidation therapy with autologous Hematopoietic cell transplantation (HCT) have reported favourable outcomes in patients with PTCL. Autologous HCT and allogenic HCT has been associated with survival benefit in patients with relapsed or refractory disease.5 Currently surgery holds no role in treatment of PTCL. Surgery is restricted mainly to the complications occurring during the course of treatment.
Chemotherapy plays an important role in the treatment of patients with NHL.6 One well-known NHL consequence that may happen at the time of initial presentation is intestinal perforation; otherwise, it may happen after chemotherapy is started. It is challenging to anticipate intestinal perforation before treatment, and there is no tried-and-true method for reducing the incidence of really unfavourable gastrointestinal events in such cases.7 The reasons for perforation in those receiving chemotherapy include fast tumour necrosis, tumour lysis and tissue impairment due to excessive granulation.8 The rate of perforation in cases of intestinal lymphoma post chemotherapy is about 9%. Small bowel is the most often perforated region in GI lymphoma cases, and diffuse large B-cell lymphoma is the most prevalent cause of perforations.9 We present a rare case where a proven Peripheral T cell lymphoma presented to us with small bowel perforation peritonitis shortly (barely within a week) after initiation of the first cycle of chemotherapy.
A little over half of the perforations following chemotherapy happened during the first month or cycle, while the other half happened more than four weeks after the chemotherapy was started. In addition to treatment-related problems such as neutropenic colitis, infectious colitis, radiation enteritis, and colonic pseudo-obstruction, these perforations also resulted from the extensive involvement of the lymphoma itself. Preoperative smoking or comorbid conditions such as diabetes mellitus, increased LDH levels, decreased albumin levels and T- cell lymphoma are considered independent risk factors for perforation in case of GI lymphomas.10 A range of time to perforation from 4 days to more than 5 weeks from the start of treatment has also been reported in other small series of perforation incidents in GI lymphoma. Perforation may occur and the clinical symptoms may go unnoticed, depending on the amount of steroids administered to the patient.11
GI perforation can be life-threatening because the patients are already debilitated and immunocompromised after receiving chemotherapy. There is conflicting evidence regarding the best surgical approach for treating GI perforation. Primary resection with formation of stoma is the preferred choice in case of generalised peritonitis and in patients with large unresectable tumour. Primary resection anastomosis is typically avoided owing to the increased risk of anastomotic leak due to immunosuppression. However, recent reports suggest that resection and primary anastomosis can be performed safely in certain surgical conditions and with sophisticated surgical techniques.12
It is still unclear how beneficial elective surgery is for treating intestinal lymphoma. Uncertainty surrounds whether removing localized illness prior to starting chemotherapy avoids later risks including bleeding and perforation. While the impact on overall survival was not statistically significant, studies have shown that patients with localized disease respond better to surgical resection followed by chemotherapy.13,14 For aggressive or multifocal disease, given the location and severity of the disease, resection prior to chemotherapy may not be both technically possible and wise given the necessary time for adequate tissue repair following the delay in chemotherapy. The choice of whether or not to resect a gastrointestinal lymphoma prior to chemotherapy is based on a number of factors, such as the disease's location and extent, its rate of progression, the existence of haemorrhage, and the possibility of long-term side effects including short-gut syndrome from resection.
Our case highlights the need for vigilance after initiating chemotherapy for detecting such life threatening complications at the earliest. We are of the firm opinion that providing a stoma in such complicated cases leads to earlier recovery and gives us an opportunity to avoid any delay in reinitiating the chemotherapy regimen.
As shown in this case, treatment for gastrointestinal lymphoma must constantly take into account the potential complications such as bleeding and perforation. While systemic chemotherapy is the mainstay of treatment for advanced lymphoma. Surgeons should be aware of possibility of such complications in patients with high risk factors. At present there is no consensus/ guidelines for elective surgery in GI lymphomas. But in few cases, surgery has been found to have limited role in excision of localised disease in refractory/ relapse cases and is mainly restricted to the complications during the course of treatment.
Notes
AUTHOR CONTRIBUTIONS
Raju Ponnusamy, Pinak Dasgupta and Ajay Pai have contributed to the conception/ design of the work; the acquisition, analysis, and interpretation of data for the work; revising the work critically for important intellectual content; including the final approval of the version to be published.
REFERENCES
1. Gou HF, Zang J, Jiang M, Yang Y, Cao D, Chen XC. 2012; Clinical prognostic analysis of 116 patients with primary intestinal non-Hodgkin lymphoma. Med Oncol. 29:227–234. DOI: 10.1007/s12032-010-9783-x. PMID: 21193968.
2. Ghimire P, Wu GY, Zhu L. 2011; Primary gastrointestinal lymphoma. World J Gastroenterol. 17:697–707. DOI: 10.3748/wjg.v17.i6.697. PMID: 21390139. PMCID: PMC3042647.
3. Shirwaikar Thomas A, Schwartz M, Quigley E. 2019; Gastrointestinal lymphoma: the new mimic. BMJ Open Gastroenterol. 6:e000320. DOI: 10.1136/bmjgast-2019-000320. PMID: 31645987. PMCID: PMC6782046.
4. Spectre G, Libster D, Grisariu S, et al. 2006; Bleeding, obstruction, and perforation in a series of patients with aggressive gastric lymphoma treated with primary chemotherapy. Ann Surg Oncol. 13:1372–1378. DOI: 10.1245/s10434-006-9069-x. PMID: 17009162.
5. T-Cell Lymphomas (Version 4.2024). [Internet]. National Comprehensive Cancer Network;Available from: https://www.nccn.org/professionals/physician_gls/pdf/t-cell.pdf. cited 2024 Jul 30.
6. Ansell SM. 2015; Non-Hodgkin lymphoma: Diagnosis and treatment. Mayo Clin Proc. 90:1152–1163. DOI: 10.1016/j.mayocp.2015.04.025. PMID: 26250731.
7. Imataki O, Shiroshita K, Uchida S, Kida J, Akamoto S, Uemura M. 2016; Perforation in an intestinal malignant lymphoma case. BMC Res Notes. 9:308. DOI: 10.1186/s13104-016-2111-6. PMID: 27297406. PMCID: PMC4906586.
8. Tatar C, Yavas M, Akkus O, et al. 2017; Intestinal perforation that developed after chemotherapy in a patient diagnosed with non-Hodgkin lymphoma: A case report and review of literature. Int J Surg Case Rep. 39:321–323. DOI: 10.1016/j.ijscr.2017.08.058. PMID: 28898795. PMCID: PMC5597875.
9. Vaidya R, Witzig TE. 2014; Incidence of bowel perforation in gastrointestinal lymphomas by location and histology. Ann Oncol. 25:1249–1250. DOI: 10.1093/annonc/mdu135. PMID: 24692578. PMCID: PMC4110448.
10. Vaidya R, Habermann TM, Donohue JH, et al. 2013; Bowel perforation in intestinal lymphoma: incidence and clinical features. Ann Oncol. 24:2439–2443. DOI: 10.1093/annonc/mdt188. PMID: 23704194. PMCID: PMC3755328.
11. Abbott S, Nikolousis E, Badger I. 2015; Intestinal lymphoma--a review of the management of emergency presentations to the general surgeon. Int J Colorectal Dis. 30:151–157. DOI: 10.1007/s00384-014-2061-1. PMID: 25374417.
12. Ahmed G, ElShafiey M, Abdelrahman H, et al. 2019; Surgery in perforated pediatric intestinal lymphoma. Eur J Surg Oncol. 45:279–283. DOI: 10.1016/j.ejso.2018.08.022. PMID: 30224248.
13. Ibrahim EM, Ezzat AA, El-Weshi AN, et al. 2001; Primary intestinal diffuse large B-cell non-Hodgkin's lymphoma: clinical features, management, and prognosis of 66 patients. Ann Oncol. 12:53–58. DOI: 10.1023/A:1008389001990. PMID: 11249049.
14. Kim SJ, Kang HJ, Kim JS, et al. 2011; Comparison of treatment strategies for patients with intestinal diffuse large B-cell lymphoma: surgical resection followed by chemotherapy versus chemotherapy alone. Blood. 117:1958–1965. DOI: 10.1182/blood-2010-06-288480. PMID: 21148334.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download