Abstract
Background/Aims
Utilization of low-volume preparation agents is crucial to improve patient willingness to undergo repeat colonoscopies. However, gastric safety data on preparation agents are limited. This study evaluated the acute gastropathy associated with bowel preparation agents.
Methods
This retrospective study enrolled healthy subjects who underwent both esophagogastroduodenoscopy and colonoscopy screening. Baseline patient characteristics, bowel preparation success, acute gastropathy, and polyp and adenoma detection rates were evaluated for 1 L polyethylene glycol with ascorbic acid (1 L PEG/Asc) and oral sulfate tablet (OST) groups.
Results
Comparison of the OST group (n=2,463) with the 1 L PEG/Asc group (n=2,060) revealed that the rates of successful cleansing and high-quality cleansing were similar between the two groups. Polyp and adenoma detection rates were significantly higher in the OST group than in the 1 L PEG/Asc group (p<0.001 and p=0.013), while the incidence of acute gastric mucosal lesion-like blood stain/clot, erosions at greater curvature side of antrum/body, multiple erosions, and overlying mucosal erythema or edema were all significantly higher in the OST group than in the 1 L PEG/Asc group (all p<0.001). Additionally, high and indeterminate probability scores of preparation agent-induced gastropathy (p=0.001) and mean Lanza scores were significantly higher in the OST group than in the 1 L PEG/Asc group (1.3 vs. 0.4, p<0.001).
Colonoscopy is the most effective tool for early detection of colorectal cancer and removal of adenomatous polyps.1,2 Adequate bowel preparation is imperative for thorough examination of the entire colonic mucosa during colonoscopy. Nevertheless, inadequate bowel preparation frequently occurs due to patient intolerance to high-volume preparation agents. Conventional preparation agents have been 4 L polyethylene glycol (PEG) and 2 L PEG with ascorbic acid (PEG/Asc); however, both agents require a total liquid intake of 3–4 L.3,4 Therefore, the availability of low-volume preparation agents is important for increasing patient willingness to undergo repeat colonoscopies.
Recently, 1 L PEG/Asc has become available as a low-volume bowel preparation agent with an efficacy and safety similar to those of 2 L PEG/Asc.5,6 Additionally, oral sulfate tablets (OSTs) have been developed with an identical chemical composition to those of oral sulfate solutions, which have a pungent flavor due to their high sulfate content.7,8 OSTs are non-inferior to 2 L PEG/Asc in terms of preparation efficacy, but it demonstrated better preparations both in the overall colon and proximal colon across randomized trials7,8 and real-world data.9 However, recent experiences with OSTs have highlighted a critical safety concern related to acute gastropathy.10 As most clinical trials for bowel preparation do not include safety data of gastric mucosa,7,8 the incidence of acute gastropathy associated with bowel preparation agents may be underestimated. Similar to pill-induced esophagitis, delayed dissolution of OSTs in the stomach may result in acute gastropathy, characterized by erosion or ulceration of the mucosal lining due to prolonged contact of the OST with the gastric mucosa. However, no study has reported regarding the safety of bowel preparation agents concerning the occurrence of acute gastropathy.
In this large observational study, we evaluated the efficacy of bowel preparation agents and incidence of acute gastropathy associated with bowel preparation in healthy subjects who underwent both esophagogastroduodenoscopy (EGD) and colonoscopy screening during routine health checkups.
This was a retrospective study of healthy subjects who underwent both screening EGD and colonoscopy at the Health Promotion Center of a single academic hospital in South Korea between January 1, 2021 and December 31, 2022. On the day of the endoscopy, the following data were collected from each patient: demographic, medication history, previous abdominal or pelvic surgery, comorbid disease, laboratory data on the day of endoscopy, EGD and colonoscopy data, quality of colon cleansing, and moderate or severe adverse effects (AEs). In this study, data of mild AEs were not considered, as they were not routinely described in electronic medical records. We compared the efficacy of bowel preparation agents and incidence of acute gastropathy associated with the type of preparation agent used (OST vs. 1 L PEG/Asc). Blood urea nitrogen (BUN), sodium, chloride, potassium, calcium, and phosphorus levels were measured on the day of endoscopy (post-preparation), and abnormal laboratory data were defined as data beyond the normal range for each variable. As laboratory data were not measured for all participants, they were analyzed only for those with the complete data.
Healthy subjects who were 18 years of age or older, underwent both screening EGD and colonoscopy on the same day, prepared with OST or 1 L PEG/Asc, and possessed sufficient medical information were included in our study. At our center, EGD and colonoscopy were not recommended for patients with the following conditions: American Society of Anesthesiologists physical status index >III; acute myocardial infarction within 24 weeks; history of significant constipation (<3 bowel movements per week with or without regular laxatives); known or suspected ileus, gastrointestinal obstruction, gastric retention, bowel perforation, toxic colitis or megacolon; active intestinal bleeding; severe renal insufficiency; a physical or mental disability that would interfere with the health check-up; pregnant or lactating women; or coronavirus disease-19 diagnosis within 1 month. The requirement for informed consent was waived for all candidates due to the retrospective nature of the study. This study was approved by the Institutional Ethics Committee (KHNMC IRB 2023-03-008) and was conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice guidelines.
At our center, bowel preparation agents were determined according to the preference of healthy subjects, and all endoscopies were performed during morning hours. The subjects were instructed to consume a low-residue diet for three days prior to their colonoscopy and restricted to a soft or liquid diet for dinner on the day before colonoscopy. The subjects were instructed to consume the preparation agents as a split-dose regimen at home; the first dose was administered at 19:00–21:00 on the night before colonoscopy, and the second dose at 05:00–08:00, within 2–3 hours ahead of the scheduled time of endoscopy. The coordinator nurse directed the use of the preparation agents. The dosing regimen of OST (Orafang®; Phambio Korea, Chungju, Korea) consisted of 28 tablets taken orally: a first sequence of 14 tablets with water and additional 1 L of water for an hour on the day before colonoscopy, and a second sequence of 14 tablets administered similarly on the morning of the colonoscopy.8,9,11 Subjects in the 1 L PEG/Asc (CleanViewAL®; Taejoon Pharm Co., Ltd., Seoul, Korea) group were instructed to drink a 500 mL solution comprising the powdered drug and water, followed by at least 500 mL water per dose.5,6 A second sequence of 1 L PEG/Asc was similarly administered on the morning of the colonoscopy. Patients who were on aspirin, antiplatelet agents, or anticoagulants were recommended to discontinue their medications according to the guidelines.
Screening EGD and colonoscopy were performed by two highly experienced, board-certified gastroenterologists who had performed 10,000 colonoscopies using a high-definition EGD and colonoscopy system (290 series; Olympus America, Center Valley, PA, USA). During colonoscopy, the quality of the bowel preparation was assessed using the Boston Bowel Preparation Scale (BBPS).12-14 At our center, bowel preparation was considered adequate if the overall BBPS score was 6 or higher.14 Successful overall bowel preparation was defined as a BBPS score ≥2 for all segments, and high-quality bowel preparation on overall or segmental evaluation was defined as a BBPS score of 3.5 Other secondary outcomes were polyp detection rate (PDR) and adenoma detection rate (ADR), which were calculated as the percentage of patients with at least one polyp (for PDR) or adenoma (for ADR) seen on colonoscopy.
Four non-investigating gastroenterologists, who were blinded to the preparation agent administered, independently reviewed and assessed all EGD findings and their possible relationships with the preparation agents using dedicated criteria under a collegial decision. A group decision was made by agreement after several rounds of discussion by each gastroenterologist for majority cases, or by majority vote for minority cases when agreement is not reached despite several rounds of discussion. They evaluated EGD findings that could be related to bowel preparation, such as acute gastropathy and fluid retention ≥200 mL. Acute gastropathy associated with bowel preparation was defined as erosive gastritis and gastric ulcerations with hematins (Fig. 1), based on a previous study for sodium phosphate (NaP) tablet-associated gastric lesions.15 Acute gastropathy was subcategorized as multiple, acute gastric mucosal lesion (AGML)-like blood hematins or mucosal erythema/edema, which generally occurs in the greater curvature (GC) of the body and antrum (at their dependent position) and has been described as drug-induced gastric lesions in other studies.15-17 Acute gastropathy was assessed using a specific grid to determine its relationship with bowel preparation (Supplementary Table 1), which was modified from the criteria used for NaP tablet-induced gastric lesions.15 In the imaging of drug-induced gastropathy, lesions generally occur in the GC of the body and antrum and are mostly dependent on the position.16 Additionally, the severity of mucosal injury was evaluated using the Lanza score;17 normal stomach=0; mucosal hemorrhage only=1; one or two erosions= 2; numerous (3–10) areas of erosion=3; and a large number of erosions (>10) or ulcers=4. Retention of food or fluid ≥200 mL was recorded on each EGD examination.
Continuous variables are presented as the mean±standard deviation, whereas categorical variables are expressed as number (percentages). Variables were compared using the chi-square test or Fisher's exact test between two groups and analysis of variance or Kruskal-Wallis test between three groups. All statistical tests were two-sided, and a p-value <0.05 was considered statistically significant. Statistical analyses were conducted using SPSS Korea plus 28.0 (SPSS Inc., Chicago, IL, USA).
Among the 4,718 patients who underwent EGD and colonoscopy screening on the same day, 4,703 were eligible for the study. Fifteen patients were excluded for using 2 L PEG/Asc. Table 1 shows the characteristics of the 2,643 participants in the OST group (56.2%) and 2,060 participants in the 1 L PEG/Asc group (43.8%). Patients in the OST group were significantly older than the 1 L PEG/Asc group (53.7 vs. 51.2 years, p<0.001). Regarding age groups, participants ≥50 years predominantly preferred OST over 1 L PEG/Asc, while participants <50 years demonstrated the opposite preference (p<0.001). Men significantly preferred 1 L PEG/Asc over OST and women demonstrated the opposite preference (p<0.001). The difference in body mass index and history of abdominal surgery between the two groups was negligible. Incidence of comorbid diseases, hypertension, diabetes mellitus, ischemic heart disease, and malignancy were similar between the two groups; however, the number of participants with cerebrovascular disease were higher in the OST group than in the 1 L PEG/Asc group (p=0.003). No significant difference was observed in the medication history, including consumption of aspirin or antiplatelet agents, anticoagulants, and nonsteroidal anti-inflammatory drugs (NSAIDs), between the two groups.
Bowel preparation efficacy are shown in Table 2. Rates of successful cleansing, high-quality cleansing, and termination of colonoscopy due to inadequate preparation did not differ between the groups. The rates of successful cleansing and high-quality cleansing were over 92% and over 51%, respectively, in both groups. The total mean BBPS score was slightly higher in the OST group than in the 1 L PEG/Asc group (7.9 vs. 7.8, p=0.040). When BBPS score was analyzed for each segment of the colon, the efficacy in the right- and left-sided colon was comparable between the two groups; however, the efficacy in the transverse colon was better in the OST group than in the 1 L PEG/Asc group, with minimal difference.
Regarding colonoscopy outcomes, cecal intubation rates were excellent in both groups (99.9%). Three patients in the OST group failed to undergo cecal intubation due to technical difficulty (n=1) and inadequate preparation (n=2), and three cases in the 1 L PEG/Asc group failed to undergo cecal intubation due to inadequate preparation (n=3). The PDR and ADR of the OST group were significantly higher than those of the 1 L PEG/Asc group (p<0.001 and p=0.013, respectively); however, the detection rate of colorectal cancer did not differ between the two groups (Table 2).
Table 3 shows the association between acute gastropathy and bowel preparations. The incidences of subcategories of acute gastropathy, including AGML-like hematins, erosions on the GC side of the antrum or body, multiple erosions, and overlying mucosal erythema or edema, were all significantly higher in the OST group than in the 1 L PEG/Asc group (all p<0.001). When acute gastropathy was classified as the probability of association with the preparation agent based on predefined criteria, high and indeterminate probability scores were significantly higher in the OST group than in the 1 L PEG/Asc group (p<0.001). The mean Lanza score was significantly higher in the OST group than in the 1 L PEG/Asc group (1.3 vs. 0.4, p<0.001). Retention of fluid ≥200 mL was significantly higher in the 1 L PEG/Asc group than in the OST group (p<0.001).
Regarding clinical safety, moderate or severe AEs were not detected in both groups. No AEs requiring hospitalization or emergency department visits were reported. The laboratory data on the day of endoscopy are presented in Table 4. Blood levels of BUN, potassium, chloride, calcium, and phosphorus were significantly higher in the 1 L PEG/Asc group than in the OST group, except for the sodium level. In addition, subjects with abnormal values beyond the normal range were detected significantly more frequently in the 1 L PEG/Asc group than in the OST group, except for the sodium level.
This is the first largest study to focus on acute gastropathy associated with bowel preparation in the OST (n=2,463) and 1 L PEG/Asc group (n=2,060). In South Korea, it is common practice to perform EGD and colonoscopy simultaneously for health checkups; consequently, this study reports acute gastropathy for both these procedures. However, it has not been reported in the USA, even though performing EGD with colonoscopy is a standard procedure in clinical practice. Furthermore, most clinical trials on bowel preparation do not include safety data on the gastric mucosa;7,8 therefore, acute gastropathy associated with bowel preparation may have been underestimated until recently. We found that the incidence of acute gastropathy associated with bowel preparation, such as AGML-like blood stains/clots, erosions on the GC side of the antrum or body, multiple erosions, and overlying mucosal erythema or edema, was significantly higher in the OST group than in the 1 L PEG/Asc group (all p<0.001). In addition, the high and indeterminate probability scores of the preparation agent association were significantly higher in the OST group than in the 1 L PEG/Asc group (p<0.001). In our study, all participants were asymptomatic, healthy participants who underwent screening EGD and colonoscopy on the same day. No significant difference was observed between the two groups in terms of medication history, including aspirin or antiplatelet agents, anticoagulants, and NSAIDs. Furthermore, gastropathy detected on EGDs is similar to pill-induced gastropathy.15-17 Therefore, a high rate of gastropathy detected on EGD on the same day as colonoscopy may be associated with bowel preparation. Our findings were consistent with a recent report from the USA which indicated a likely association between erosive gastritis and OST use when compared to oral liquid preparations; however, previous reports were limited by a small sample size.10 Similar gastric lesions have been reported for NaP tablets in humans and pigs.15,18 Our findings highlight that physicians should focus on gastropathy associated with bowel preparation, especially in OST users, as it could interfere with EGD findings.
We also found that older participants (≥50 years) and women significantly preferred OSTs to 1 L PEG/Asc (both p<0.001). Older participants may prefer OSTs due to bad experiences with liquid preparation agents in past colonoscopies, while women may prefer OSTs due to their higher sensitivity to a pungent flavor than men. In a randomized controlled trial comparing OSTs with 1 L PEG/Asc,19 the participants’ preference for the preparation agent could not be evaluated by the nature of randomization. Therefore, there have been no data on the preference for OST vs. 1 L PEG/Asc. This study provides information on patient’s preferences for the preparation agent between OST vs. 1 L PEG/Asc. Considering that a significant number of patients still choose over-the-counter preparation agents for colonoscopy in the USA,20,21 physicians need to focus on patients’ preferences for bowel preparation. For examples, 1,137,810 (56.5%) of 2.8 million participants used over-the-count preparation agents for screening colonoscopies,20 and 56,496 (43%) also used over-the-count preparation agents for screening colonoscopies in medicare population.21
Our real-world data indicates high efficacy of the preparation with OSTs and 1 L of PEG/Asc as successful cleansing was over 92% in both groups. Our efficacy levels in real-world data were similar to those in a recent clinical trial comparing OST and 1 L PEG/Asc,18 in which the rates of successful cleansing with OSTs and 1 L PEG/Asc were 95% and 96%, respectively. In our study, the total mean BBPS score was slightly higher in the OST group than in the 1 L PEG-Asc group (7.9 vs. 7.8, p=0.040), and ADR was significantly higher in the OST group than in the 1 L PEG-Asc group (p=0.013). Our findings were similar to other real-world data (n=992) comparing OST with 2 L PEG/Asc,9 in that the total mean BBPS scores were slightly higher in the OST group than in the 2 L PEG/Asc group (8.2 vs. 7.8, p=0.014), and ADR was significantly higher in the OST group than in the 2 L PEG/Asc group (38.6% vs. 27.1%, p=0.003). However, in previous clinical trials, the efficacy of preparation and ADR did not differ between OST and 1 L PEG/Asc19 or 2 L PEG/Asc.8,11 Real-world data, including our study, may yield different results from those of clinical trials for ADR, as real-world data is based on larger sample sizes compared to clinical trials and subjects may exhibit higher levels of compliance with OST than with liquid agents in real- world. However, higher ADR in OST group may also be influenced by older age in OST group than 1 L PEG/Asc group (53.7 vs. 51.2 years, p<0.001).
Our study is the first and largest study to focus on acute gastropathy associated with bowel preparation. Despite the retrospective design, our participants were all asymptomatic, healthy individuals who underwent screening endoscopy. In addition, detailed clinical information, including comorbidities and medication history, was available from the health check-up data. Despite these strengths, this study has several limitations. First, due to the lack of a standard definition of acute gastropathy associated with bowel preparation agents, we used modified criteria for NaP tablet-induced gastric lesions.15 Although this grid is not a standardized and validated tool for assessing the causal association between acute gastropathy and bowel preparation agents,15 EGD findings of these lesions are typical (Fig. 1), and this grid considers ulcerogenic medication as well as EGD findings. Second, the possibility of selection bias for bowel preparation agent preference cannot be excluded, although it was chosen based on patient preference. To exclude potential biases, a large-scale randomized controlled trial is warranted. Third, baseline characteristics of the study population may differ from those of the general population. As our subjects were relatively healthy persons from ‘Health Promotion Center’ of a single center, a multi-center study from general population may be necessary. However, it is not free from this potential bias, even if performed in a randomized controlled trial. Fourth, we compared the efficacy of and gastropathy associated with OSTs and 1 L PEG/Asc only; therefore, other liquid preparation agents should be compared. Finally, owing to the retrospective nature of the study, we could not capture mild AEs or baseline laboratory data before endoscopy. Moreover, laboratory data on the day of endoscopy were missing, however, these have been well studied in previous clinical trials8,11,19 and were not the focus of this study. Large-scale prospective studies may better demonstrate the safety profiles of bowel preparations. Considering that EGD and colonoscopy screening are often performed simultaneously in Korea, preparation- associated gastropathy may negatively impact on the EGD screening for the detection of early gastric cancer or Helicobacter pylori examination. Therefore, further research on these topics is also necessary.
In conclusion, our large-scale study showed that OSTs were more associated with acute gastropathy compared to 1 L PEG/Asc, but had a comparable efficacy. Physicians should pay attention to gastropathy associated with preparation agents, especially for OST users, and to patient preferences when recommending preparation agents for colonoscopy.
Supplementary material is available at the Korean Journal of Gastroenterology website (https://www.kjg.or.kr/).
ACKNOWLEDGEMENTS
We thank the four safety committee gastroenterologists: HS Kang, SH Jeon, MA Suh, and YK Kim.
REFERENCES
1. Nishihara R, Wu K, Lochhead P, et al. 2013; Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med. 369:1095–1105. DOI: 10.1056/NEJMoa1301969. PMID: 24047059. PMCID: PMC3840160.
2. Løberg M, Kalager M, Holme Ø, Hoff G, Adami HO, Bretthauer M. 2014; Long-term colorectal-cancer mortality after adenoma removal. N Engl J Med. 371:799–807. DOI: 10.1056/NEJMoa1315870. PMID: 25162886.
3. Cohen LB, Sanyal SM, Von Althann C, et al. 2010; Clinical trial: 2-L polyethylene glycol-based lavage solutions for colonoscopy preparation- a randomized, single-blind study of two formulations. Aliment Pharmacol Ther. 32:637–644. DOI: 10.1111/j.1365-2036.2010.04390.x. PMID: 20626383.
4. Hassan C, East J, Radaelli F, et al. 2019; Bowel preparation for colonoscopy: European Society of Gastrointestinal Endoscopy (ESGE) guideline - update 2019. Endoscopy. 51:775–794. DOI: 10.1055/a-0959-0505. PMID: 31295746.
5. Jung Y, Kang SB, Yoon HJ, Cha JM. 2022; Improving the tolerability and safety of 1-L polyethylene glycol plus low-dose ascorbic acid for bowel preparation in a healthy population: a randomized multicenter clinical trial. Gastrointest Endosc. 96:341–350.e1. DOI: 10.1016/j.gie.2022.03.007. PMID: 35288148.
6. Yoon JY, Kim HG, Cho YS, Kim HI, Cha JM. 2022; 1 L- versus 2 L-polyethylene glycol with ascorbic acid for bowel preparation in elderly patients: a randomized multicenter study. Surg Endosc. 36:5724–5733. DOI: 10.1007/s00464-021-08947-4. PMID: 35031868.
7. Di Palma JA, Bhandari R, Cleveland MV, et al. 2021; A safety and efficacy comparison of a new sulfate-based tablet bowel preparation versus a PEG and ascorbate comparator in adult subjects undergoing colonoscopy. Am J Gastroenterol. 116:319–328. DOI: 10.14309/ajg.0000000000001020. PMID: 33165006. PMCID: PMC7864663.
8. Kim JH, Park YE, Kim TO, et al. Comparison of the efficacy and safety between oral sulfate tablet and polyethylene glycol for bowel preparation before colonoscopy according to age. Medicine (Baltimore). 2022; 101:e29884. DOI: 10.1097/MD.0000000000029884. PMID: 35801801. PMCID: PMC9259131.
9. Lee SE, Oh DJ, Nam JH, et al. 2023; Taking oral sulfate tablets with simethicone for bowel preparation leads to higher adenoma detection rate than polyethylene glycol: a propensity score analysis. Dig Dis Sci. 68:867–876. DOI: 10.1007/s10620-022-07611-8. PMID: 35781655.
10. Villa E, Bansal M, Zakko W. 2021; Oral sulfate tablet bowel preparation is associated with erosive gastritis. Am J Gastroenterol. 116:S263. DOI: 10.14309/01.ajg.0000774788.09911.e3.
11. Yang HJ, Park DI, Park SK, et al. 2020; Novel sulfate tablet PBK-1701TC versus oral sulfate solution for colon cleansing: a randomized phase 3 trial. J Gastroenterol Hepatol. 35:29–36. DOI: 10.1111/jgh.14826. PMID: 31396995.
12. Lai EJ, Calderwood AH, Doros G, Fix OK, Jacobson BC. 2009; The Boston bowel preparation scale: a valid and reliable instrument for colonoscopy-oriented research. Gastrointest Endosc. 69(3 Pt 2):620–625. DOI: 10.1016/j.gie.2008.05.057. PMID: 19136102. PMCID: PMC2763922.
13. Calderwood AH, Jacobson BC. 2010; Comprehensive validation of the Boston Bowel Preparation Scale. Gastrointest Endosc. 72:686–692. DOI: 10.1016/j.gie.2010.06.068. PMID: 20883845. PMCID: PMC2951305.
14. Kwak MS, Cha JM, Yang HJ, et al. 2019; Safety and efficacy of low-volume preparation in the elderly: oral sulfate solution on the day before and split-dose regimens (SEE SAFE) study. Gut Liver. 13:176–182. DOI: 10.5009/gnl18214. PMID: 30400725. PMCID: PMC6430430.
15. Hagège H, Laugier R, Nahon S, Coulom P, Isnard-Bagnis C, Albert-Marty A. 2015; Real-life conditions of use of sodium phosphate tablets for colon cleansing before colonoscopy. Endosc Int Open. 3:E346–E353. DOI: 10.1055/s-0034-1391847. PMID: 26357680. PMCID: PMC4554515.
16. McGettigan MJ, Menias CO, Gao ZJ, Mellnick VM, Hara AK. 2016; Imaging of drug-induced complications in the gastrointestinal system. Radiographics. 36:71–87. DOI: 10.1148/rg.2016150132. PMID: 26761532.
17. Lanza FL, Collaku A, Liu DJ. 2018; Endoscopic comparison of gastroduodenal injury with over-the-counter doses of new fast-dissolving ibuprofen and paracetamol formulations: a randomized, placebo-controlled, 4-way crossover clinical trial. Clin Exp Gastroenterol. 11:169–177. DOI: 10.2147/CEG.S153231. PMID: 29713191. PMCID: PMC5907787.
18. Coron E, Dewitte M, Aubert P, Musquer N, Neunlist M, Bruley des Varannes S. 2015; Reversibility of gastric mucosal lesions induced by sodium phosphate tablets and characterized by probe-based confocal laser endomicroscopy. Endosc Int Open. 3:E69–E75. DOI: 10.1055/s-0034-1377934. PMID: 26134776. PMCID: PMC4423282.
19. Park JH, Hong SW, Hwang SW, et al. 2023; Efficacy and safety of oral sodium sulfate tablet compared with 1-L polyethylene glycol plus ascorbate: a prospective, randomized, endoscopist-blinded trial. J Gastroenterol Hepatol. 38:2090–2096. DOI: 10.1111/jgh.16343. PMID: 37655723.
20. Sacks NC, Sharma A, Cyr PL, Bertiger G, Dahdal DN, Brogadir SP. 2018; Real-world comparison of the effectiveness and safety of different bowel preparation agents. Clin Exp Gastroenterol. 11:289–299. DOI: 10.2147/CEG.S171861. PMID: 30555250. PMCID: PMC6280884.
21. Pyenson B, Scammell C, Broulette J. 2014; Costs and repeat rates associated with colonoscopy observed in medical claims for commercial and medicare populations. BMC Health Serv Res. 14:92. DOI: 10.1186/1472-6963-14-92. PMID: 24572047. PMCID: PMC3974039.
Fig. 1
Example of acute gastropathy associated with bowel preparation, such as acute gastric mucosal lesion-like blood clots (A) or superficial gastric ulceration (B).
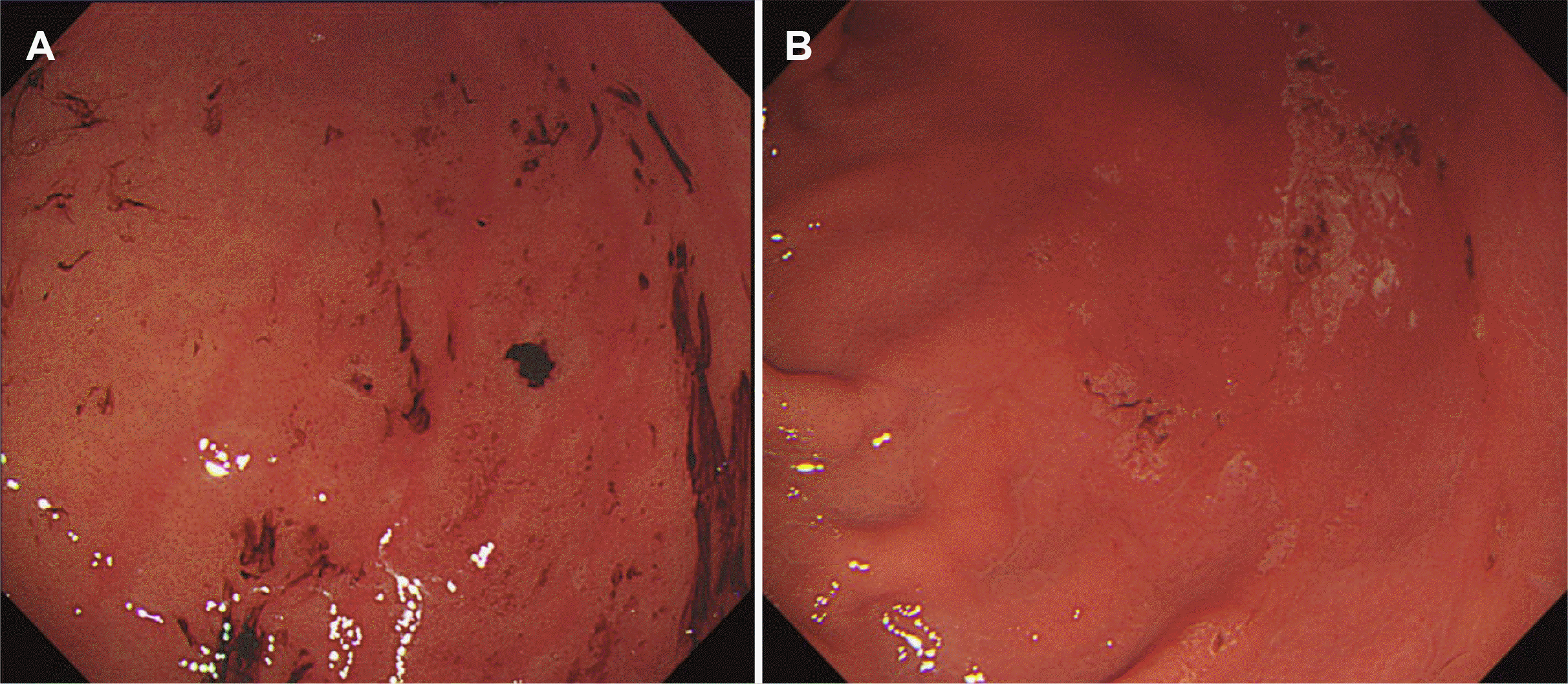
Table 1
Baseline Characteristics of Study Subjects
Table 2
Bowel Preparation Efficacy and Colonoscopy Outcomes
Table 3
Acute Gastropathy on Esophagogastroduodenoscopy
| Esophagogastroduodenoscopy outcome | Oral sulfate tablet (n=2,643) | 1 L PEG/Asc (n=2,060) | p-value |
|---|---|---|---|
| Acute gastropathya | |||
| AGML-like blood stain/clot | 458 (17.3) | 98 (4.8) | <0.001 |
| Erosions at GC side of antrum/body | 131 (39.0) | 232 (11.3) | <0.001 |
| Multiple erosions | 971 (36.7) | 245 (11.9) | <0.001 |
| Overlying mucosal erythema or edema | 272 (10.3) | 66 (3.2) | <0.001 |
| Probability score | <0.001 | ||
| High score (≥4) | 535 (20.2) | 108 (5.2) | |
| Indeterminate score (2–3) | 525 (19.9) | 147 (7.1) | |
| Low score (≤1) | 1,583 (59.9) | 1,801 (87.4) | |
| Total Lanza score | 1.3±1.5 | 0.4±1.0 | <0.001 |
| Retention of fluid ≥200 mL | 163 (4.9) | 195 (9.5) | <0.001 |
Table 4
Laboratory Data with Bowel Preparation
| Laboratory dataa | Oral sulfate tablet (n=2,651) | 1 L PEG/Asc (n=2,060) | p-value | p-value |
|---|---|---|---|---|
| BUN (mg/dL) | 12.6±3.8 | 14.7±4.3 | <0.001 | |
| Beyond NR (6–20) | 86/2,642 (3.3) | 156/2,055 (7.6) | <0.001 | |
| Sodium (mEq/L) | 140.7±3.7 | 141.5±2.4 | 0.243 | |
| Beyond NR (136–145) | 119/2,463 (4.8) | 37/811 (4.6) | 0.755 | |
| Potassium (mEq/L) | 4.4±0.4 | 4.6±0.4 | 0.002 | |
| Beyond NR (3.5–5.1) | 75/2,463 (3.0) | 58/811 (7.2) | <0.001 | |
| Chloride (mEq/L) | 102.2±3.5 | 108.8±3.4 | <0.001 | |
| Beyond NR (98–107) | 168/2,453 (6.8) | 201/302 (66.6) | <0.001 | |
| Calcium (mg/dL) | 9.8±0.4 | 10.0±0.5 | <0.001 | |
| Beyond NR (9.1–10.6) | 109/2,641 (4.1) | 192/2,054 (9.3) | <0.001 | |
| Phosphorus (mg/dL) | 3.5±0.6 | 4.1±0.6 | 0.003 | |
| Beyond NR (2.5–4.5) | 168/2,642 (6.4) | 412/2,055 (20.0) | <0.001 |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download