Abstract
Treatment patterns and preferences for patients with Graves’ disease (GD) vary across countries. In this study, we assessed the initial therapies and subsequent treatment modalities employed for GD in real-world clinical practice in Korea. We analyzed 452,001 patients with GD from 2004 to 2020, obtained from the Korean National Health Insurance Service database. Initial treatments included antithyroid drug (ATD) therapy (98% of cases), thyroidectomy (1.3%), and radioactive iodine (RAI) therapy (0.7%). The rates of initial treatment failure were 58.5% for ATDs, 21.3% for RAI, and 2.1% for thyroidectomy. Even among cases of ATD treatment failure or recurrence, the rates of RAI therapy remained low. Regarding initial treatment, the 5-year remission rate was 46.8% among patients administered ATDs versus 91.0% among recipients of RAI therapy; at 10 years, these rates were 59.2% and 94.0%, respectively. Our findings highlight a marked disparity in the use of RAI therapy in Korea compared to Western countries. Further research is required to understand the reasons for these differences in treatment patterns.
Graves’ disease (GD) is the most common form of hyperthyroidism, characterized by the excessive production of thyroid hormones due to thyroid-stimulating autoantibodies [1-3]. In Korea, the age-standardized incidence rates of hyperthyroidism were 42.23 per 100,000 men and 105.13 per 100,000 women between 2003 and 2018, with the global prevalence of GD ranging from 0.1% to 2.9% [1,2,4]. The primary treatments for GD are conventional antithyroid drugs (ATDs), radioactive iodine (RAI) therapy, and surgical thyroidectomy. However, treatment preferences vary widely across countries [5,6]. In South Korea, ATD therapy is commonly used as the initial treatment, with a recent study demonstrating that approximately 93.7% of patients with hyperthyroidism were prescribed ATD [4]. Although ATD is widely used, RAI therapy and thyroidectomy are considered alternative treatment options for patients who experience treatment failure or recurrence, as ATD therapy has a relatively high failure rate [1,3,7]. Recent studies have suggested the safety and effectiveness of long-term ATD therapy, even after treatment failure [8,9]. In a large long-term GD cohort, Jin et al. [9] found that 95% of patients received a second course of ATD treatment, with a remission rate of 54% after the first recurrence. However, the study was limited by its single-center design. Consequently, we conducted the largest retrospective nationwide GD cohort study in Korea, examining treatment patterns and preferences from initiation to the management of failure or recurrence after ATD or RAI therapy.
Using the Korean National Health Insurance Service-National Health Information Database (NHIS-NHID, number: NHIS-2022-1-208), we initially identified 641,203 patients diagnosed with GD who had received at least one of three treatments (ATDs, RAI, or thyroidectomy) following diagnosis, as per the International Classification of Diseases, 10th revision (ICD-10) code E05, between January 1, 2002 and June 30, 2020. The ATD group was defined as patients who received ATDs at least twice for ≥90 days. We excluded patients who were younger than 18 years, had a GD diagnosis in 2002 to 2003, had thyroid cancer (ICD-10 C73), had previously undergone RAI treatment or thyroidectomy, or had a follow-up duration of less than 6 months. After applying these criteria, 452,001 patients with GD were included in the cohort. Among those treated with ATD therapy, treatment failure was defined as continuous use of ATDs for more than 2 years without interruption, or the need for subsequent RAI or thyroidectomy. For the RAI group, treatment failure was defined as receiving additional RAI therapy, undergoing subsequent thyroidectomy, or initiating ATD for ≥90 days. In both groups, remission was defined as the absence of additional treatment for 12 months following initial or subsequent treatment. Censoring occurred if treatment was ongoing at the end of the study or had ceased within 1 year of treatment cessation. The baseline for each figure refers to the treatment initiation point for patients who received either ATD or RAI as their first-line therapy following a GD diagnosis. Additionally, the follow-up period was measured sequentially according to the aforementioned treatment failure, remission, or censoring criteria and was truncated at 2-year intervals. To minimize false-positive detection of RAI therapy, we specified that RAI treatment for GD must involve the prescription of at least 5 mCi in a single dose.
This study received approval from the Institutional Review Board of Korea University Anam Hospital (IRB number: 2021 AN0339). Informed consent was waived because the study relied on data from the NHIS database, which were fully anonymized and de-identified prior to analysis. All statistical analyses were conducted using SAS Enterprise Guide version 7.1 (SAS Institute Inc., Cary, NC, USA) and R version 4.1 (R Foundation for Statistical Computing, Vienna, Austria).
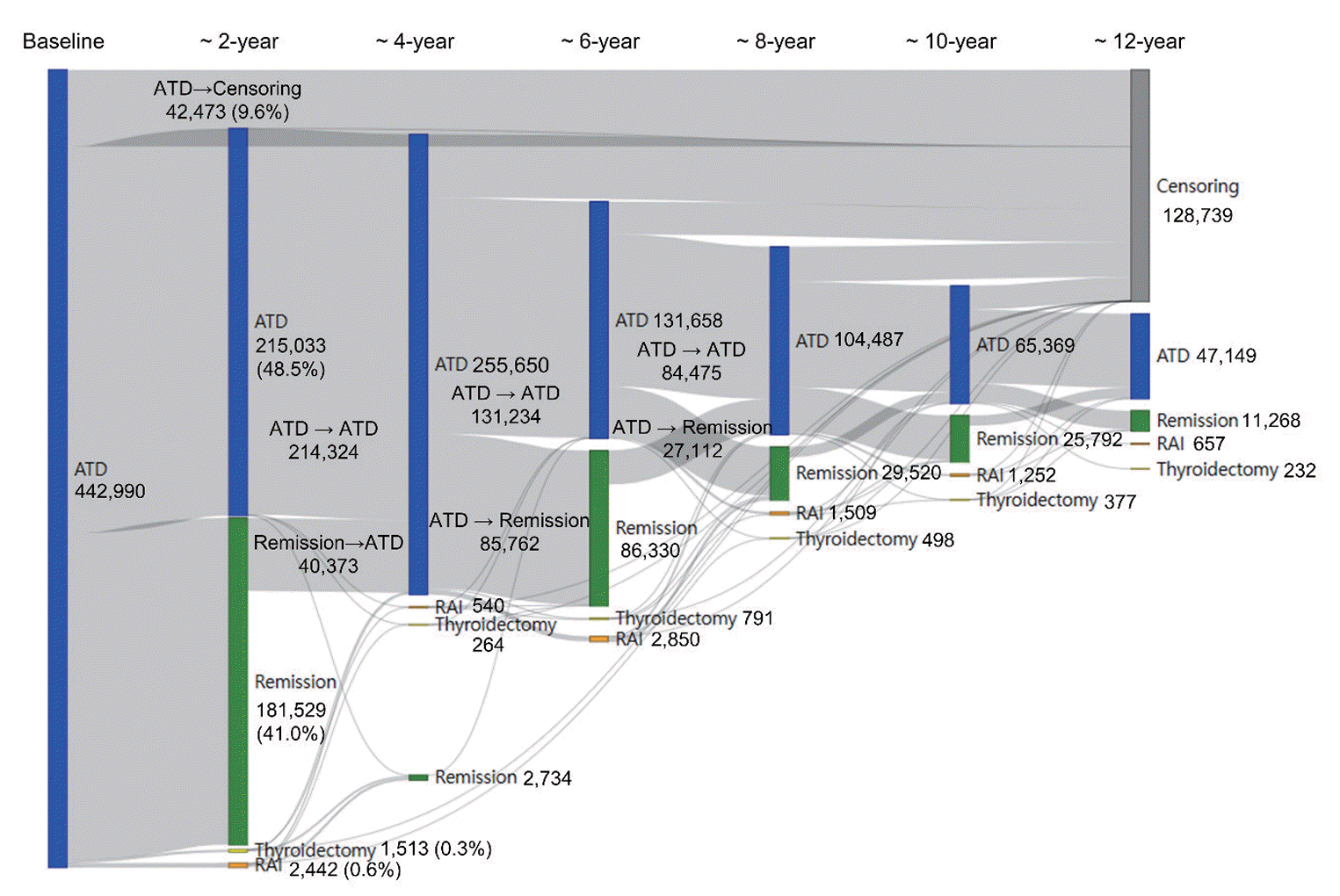
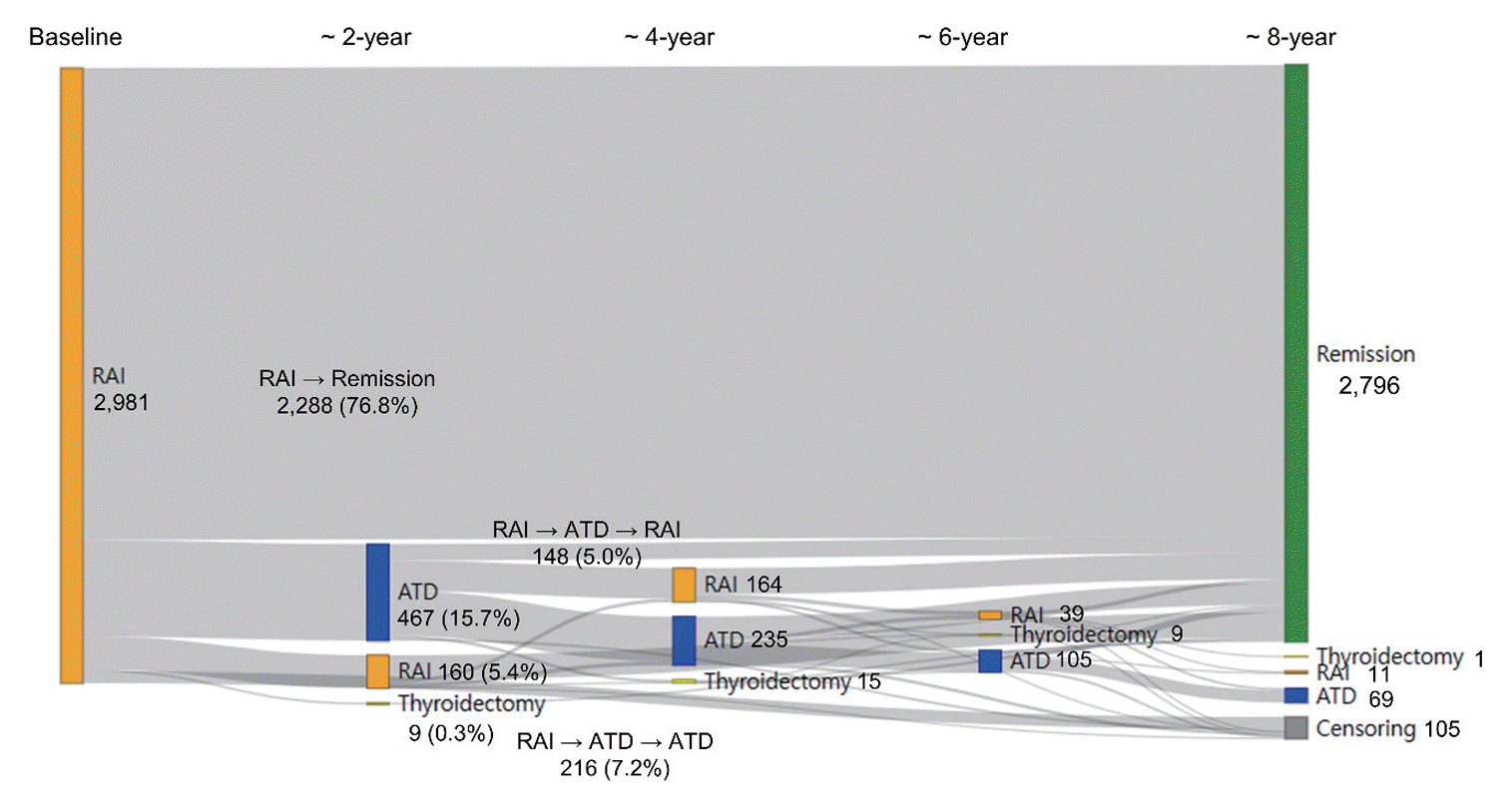
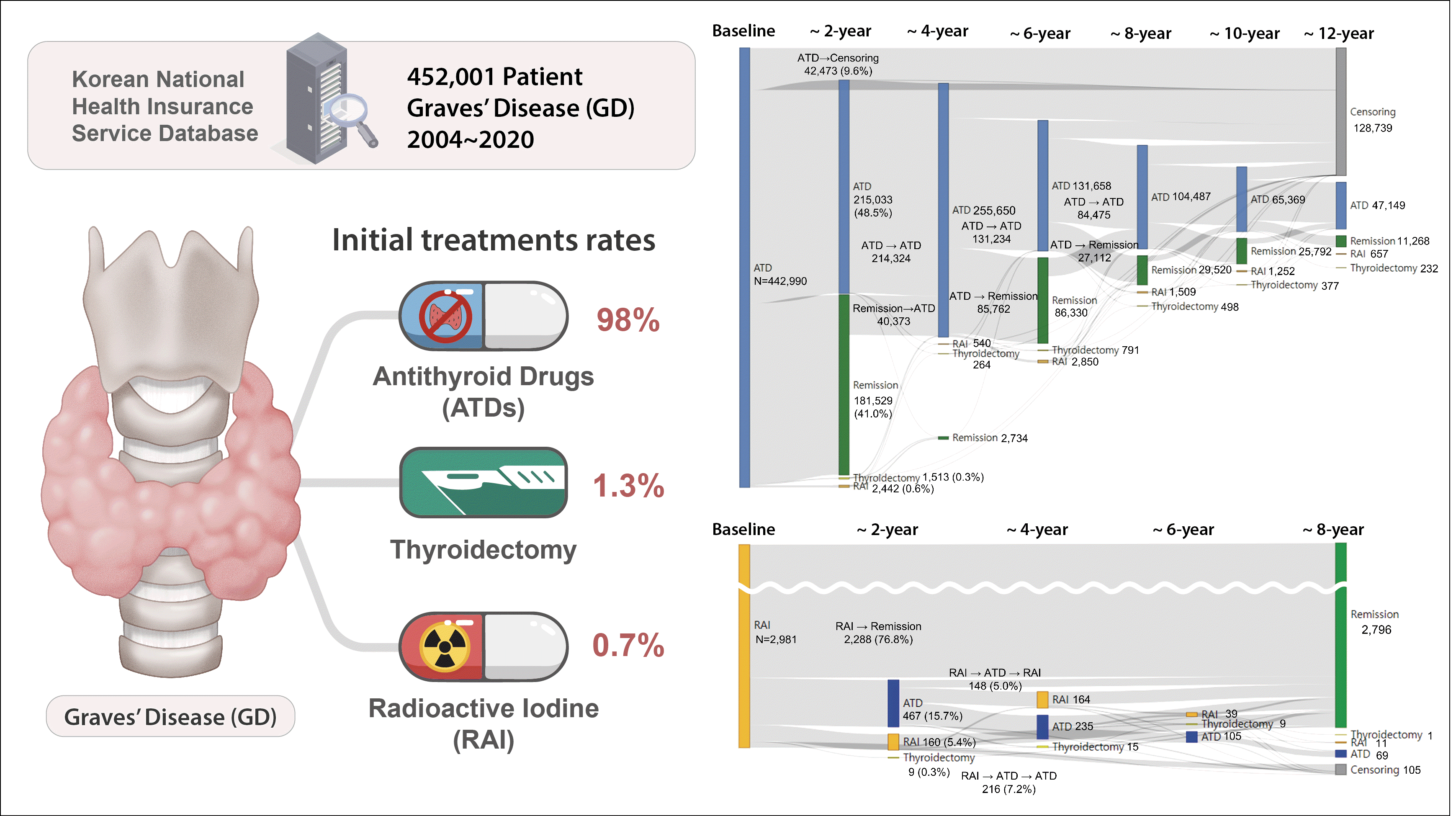
Regarding the initial treatment regimen, 98.0% of the patients with GD received ATDs, 1.3% underwent surgery, and 0.7% were given RAI therapy (Supplemental Fig. S1). The mean ages in the initial ATD and RAI groups were 46.2±14.5 and 47.2±14.6 years, respectively. Within these groups, 312,782 (70.6%) and 2,278 (76.5%) of the patients were female, with median follow-up durations of 8.5 years (interquartile range [IQR], 4.5 to 12.5) and 10.5 years (IQR, 5.9 to 14.3), respectively. Among those who received ATDs as their initial treatment, 41.0% (181,529/442,990) achieved remission after the first course of therapy (Fig. 1). Additionally, 48.5% (215,033/442,990) continued ATD therapy for more than 2 years, while 0.6% (n=2,442) received RAI therapy and 0.3% (n=1,513) underwent thyroidectomy as secondary approaches. Among the patients achieving initial remission, 40,373 experienced relapse and required reinitiation of ATDs. A total of 31,527 patients, or 7.1% of the initial ATD group, could not discontinue ATD therapy for over 10 years. The rate of RAI therapy remained very low, even among patients with ATD treatment failure or recurrence. In the initial RAI group, the median initial dose of RAI was 10 mCi (IQR, 10 to 15), with a cumulative median dose of 11 mCi (IQR, 10 to 15). A majority, 76.8% (2,288/2,981), achieved remission without further treatment. However, a total of 467 patients either could not discontinue ATDs for more than 6 months following RAI treatment (n=307) or restarted ATDs more than 6 months after RAI therapy (n=160) (Fig. 2). Only 160 patients (5.4%) received secondary RAI therapy. Ultimately, the final remission rates were 62.6% (n=277,239) in the initial ATD group and 95.2% (n=2,839) in the initial RAI therapy group, with rates of 46.8% versus 91.0% at 5 years and 59.2% versus 94.0% at 10 years, respectively.
In this nationwide cohort study, we identified ATD as the most prevalent initial treatment modality, followed by thyroidectomy and RAI therapy. To our knowledge, this is the largest nationwide cohort study to examine treatment patterns and trends among patients with GD. The initial remission rates were 41.0% for the ATD group and 76.8% for the RAI group. We observed that the use of RAI therapy was relatively infrequent, even among cases of ATD treatment failure. In comparison, after initial RAI therapy, ATD was commonly administered in patients with RAI failure.
The use of RAI therapy for GD is declining. This trend may be attributed to several factors, including concerns about potential treatment-related adverse effects such as an increased risk of cancer, exacerbation of thyroid eye disease, sialadenitis, delays in outpatient clinic appointments, and a negative perception of radiation therapy. Some physicians suggest that the hypothyroidism that often follows RAI therapy complicates its characterization as a cure for hyperthyroidism, given that it necessitates lifelong thyroid hormone replacement therapy. Nonetheless, our findings have led us to reconsider whether RAI therapy is being appropriately administered to those who need it, particularly patients whose disease is not sufficiently managed with ATDs.
This study had several limitations. For instance, we lacked access to biochemical laboratory results for thyroid function tests and antibodies, implicating the potential for false-positive diagnoses of GD and treatment failure. Another notable limitation was the relatively short follow-up period, which did not fully cover the natural course of GD treatment and recurrence. Nevertheless, the study’s primary strengths lie in its substantial sample size and population-based design, along with the accurate identification of RAI therapy and minimal loss to follow-up.
In conclusion, ATD therapy was the most common treatment for both initial and relapsed cases of GD, with a meaningful proportion of patients remaining on ATD therapy for over a decade. While further research is necessary, our findings offer valuable insights into the utilization of RAI therapy in the management of GD.
Supplementary Material
Supplemental Fig. S1.
Study design and initial treatment distribution. RAI, radioactive iodine; ATD, antithyroid drug.
ACKNOWLEDGMENTS
This research was supported by a grant from the Korean Health Technology R&D Project through the Korea Health Industry Development Institute, funded by the Ministry of Health & Welfare, South Korea (grant number: HC21C0078). The funding sources had no role in the design and conduct of the study; the collection, management, analysis, or interpretation of the data; the preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
REFERENCES
1. Ross DS, Burch HB, Cooper DS, Greenlee MC, Laurberg P, Maia AL, et al. 2016 American Thyroid Association guidelines for diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis. Thyroid. 2016; 26:1343–421.

2. Bartalena L. Diagnosis and management of Graves disease: a global overview. Nat Rev Endocrinol. 2013; 9:724–34.
3. Yi KH, Moon JH, Kim IJ, Bom HS, Lee J, Chung WY, et al. The diagnosis and management of hyperthyroidism consensus-report of the Korean Thyroid Association. J Korean Thyroid Assoc. 2013; 6:1–11.

4. Ahn HY, Cho SW, Lee MY, Park YJ, Koo BS, Chang HS, et al. Prevalence, treatment status, and comorbidities of hyperthyroidism in Korea from 2003 to 2018: a nationwide population study. Endocrinol Metab (Seoul). 2023; 38:436–44.

5. Burch HB, Burman KD, Cooper DS. A 2011 survey of clinical practice patterns in the management of Graves’ disease. J Clin Endocrinol Metab. 2012; 97:4549–58.

6. Parameswaran R, de Jong MC, Kit JLW, Sek K, Nam TQ, Thang TV, et al. 2021 Asia-Pacific Graves’ disease consortium survey of clinical practice patterns in the management of Graves’ disease. Endocrine. 2023; 79:135–42.

7. Kahaly GJ, Bartalena L, Hegedus L, Leenhardt L, Poppe K, Pearce SH. 2018 European Thyroid Association guideline for the management of Graves’ hyperthyroidism. Eur Thyroid J. 2018; 7:167–86.
Fig. 1.
Treatment pathway following initial antithyroid drug (ATD) therapy. The horizontal axis indicates the duration of follow-up, truncated at 12 years. RAI, radioactive iodine.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download