1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021; 71:209–49.

2. Sanders EM Jr, LiVolsi VA, Brierley J, Shin J, Randolph GW. An evidence-based review of poorly differentiated thyroid cancer. World J Surg. 2007; 31:934–45.
3. Sakamoto A, Kasai N, Sugano H. Poorly differentiated carcinoma of the thyroid: a clinicopathologic entity for a highrisk group of papillary and follicular carcinomas. Cancer. 1983; 52:1849–55.

4. DeLellis RA, Lloyd RV, Heitz PU, Eng C. Pathology and genetics of tumours of endocrine organs. 3rd ed. Geneva: World Health Organization;2004.
5. Hiltzik D, Carlson DL, Tuttle RM, Chuai S, Ishill N, Shaha A, et al. Poorly differentiated thyroid carcinomas defined on the basis of mitosis and necrosis: a clinicopathologic study of 58 patients. Cancer. 2006; 106:1286–95.

6. Volante M, Collini P, Nikiforov YE, Sakamoto A, Kakudo K, Katoh R, et al. Poorly differentiated thyroid carcinoma: the Turin proposal for the use of uniform diagnostic criteria and an algorithmic diagnostic approach. Am J Surg Pathol. 2007; 31:1256–64.

7. Lloyd RV, Osamura RY, Kloppel G, Rosai J. WHO classification of tumours of endocrine organs. 4th ed. Lyon: IARC;2017. Chapter 2, Tumours of the thyroid gland; p. 65-144.
8. Jung CK, Bychkov A, Kakudo K. Update from the 2022 World Health Organization classification of thyroid tumors: a standardized diagnostic approach. Endocrinol Metab (Seoul). 2022; 37:703–18.

9. Ibrahimpasic T, Ghossein R, Shah JP, Ganly I. Poorly differentiated carcinoma of the thyroid gland: current status and future prospects. Thyroid. 2019; 29:311–21.

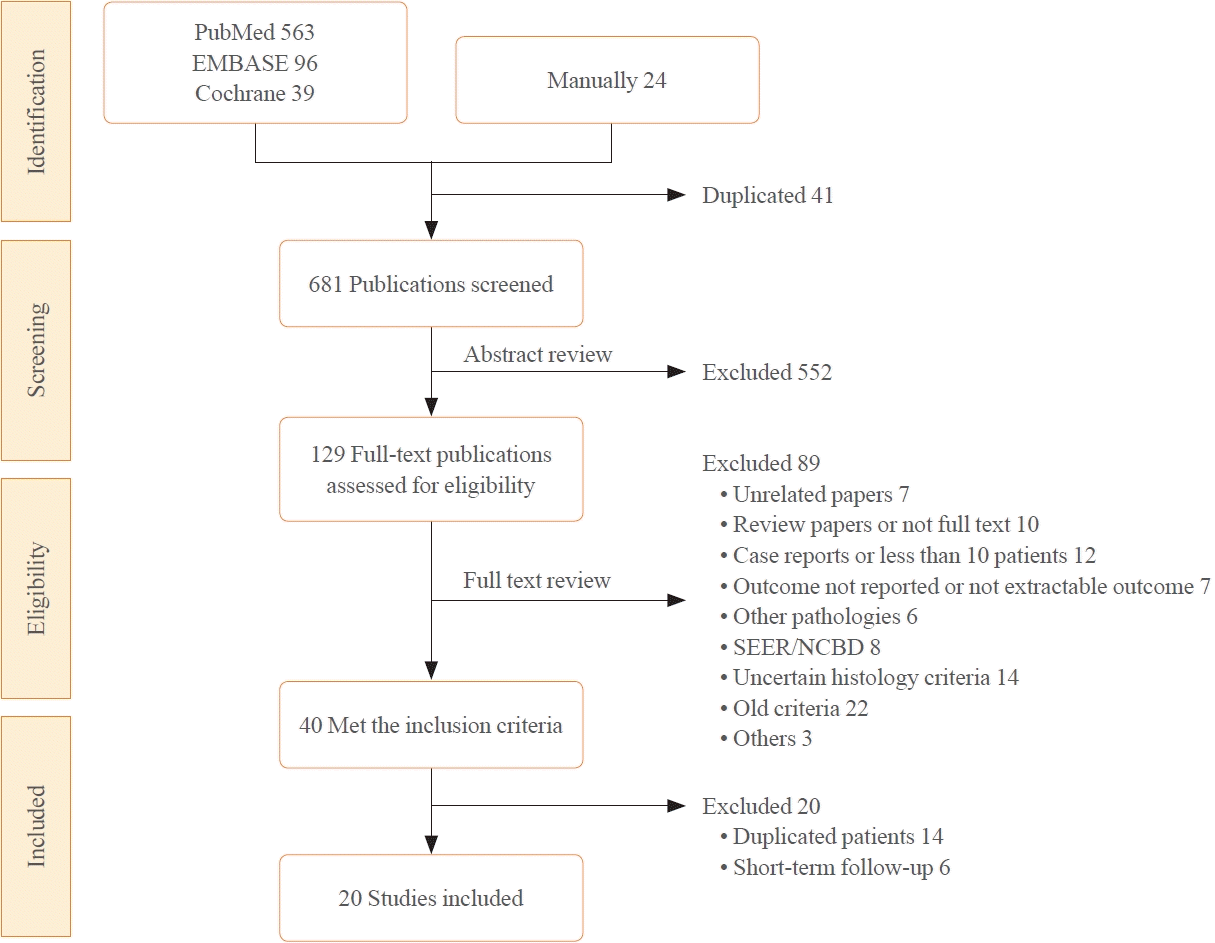
10. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021; 372:n71.
11. Carcangiu ML, Zampi G, Rosai J. Poorly differentiated (“insular”) thyroid carcinoma: a reinterpretation of Langhans’ “wuchernde Struma”. Am J Surg Pathol. 1984; 8:655–68.
12. Hayden JA, van der Windt DA, Cartwright JL, Cote P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med. 2013; 158:280–6.
13. Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010; 36:1–48.
14. Balduzzi S, Rucker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. 2019; 22:153–60.

15. Wickham H, Averick M, Bryan J, Chang W, McGowan LD, Francois R, et al. Welcome to the Tidyverse. J Open Source Softw. 2019; 4:1686.

16. Lin JD, Chao TC, Hsueh C. Clinical characteristics of poorly differentiated thyroid carcinomas compared with those of classical papillary thyroid carcinomas. Clin Endocrinol (Oxf). 2007; 66:224–8.

17. Jung TS, Kim TY, Kim KW, Oh YL, Park DJ, Cho BY, et al. Clinical features and prognostic factors for survival in patients with poorly differentiated thyroid carcinoma and comparison to the patients with the aggressive variants of papillary thyroid carcinoma. Endocr J. 2007; 54:265–74.

18. Asioli S, Erickson LA, Righi A, Jin L, Volante M, Jenkins S, et al. Poorly differentiated carcinoma of the thyroid: validation of the Turin proposal and analysis of IMP3 expression. Mod Pathol. 2010; 23:1269–78.

19. Bhargav PR, Mishra A, Agarwal G, Agarwal A, Pradhan PK, Gambhir S, et al. Long-term outcome of differentiated thyroid carcinoma: experience in a developing country. World J Surg. 2010; 34:40–7.

20. Hod R, Bachar G, Sternov Y, Shvero J. Insular thyroid carcinoma: a retrospective clinicopathologic study. Am J Otolaryngol. 2013; 34:292–5.

21. Gnemmi V, Renaud F, Do Cao C, Salleron J, Lion G, Wemeau JL, et al. Poorly differentiated thyroid carcinomas: application of the Turin proposal provides prognostic results similar to those from the assessment of high-grade features. Histopathology. 2014; 64:263–73.

22. Lee DY, Won JK, Lee SH, Park DJ, Jung KC, Sung MW, et al. Changes of clinicopathologic characteristics and survival outcomes of anaplastic and poorly differentiated thyroid carcinoma. Thyroid. 2016; 26:404–13.

23. Skansing DB, Londero SC, Asschenfeldt P, Larsen SR, Godballe C. Nonanaplastic follicular cell-derived thyroid carcinoma: mitosis and necrosis in long-term follow-up. Eur Arch Otorhinolaryngol. 2017; 274:2541–8.

24. Yu MG, Rivera J, Jimeno C. Poorly differentiated thyroid carcinoma: 10-year experience in a Southeast Asian population. Endocrinol Metab (Seoul). 2017; 32:288–95.

25. de la Fouchardiere C, Decaussin-Petrucci M, Berthiller J, Descotes F, Lopez J, Lifante JC, et al. Predictive factors of outcome in poorly differentiated thyroid carcinomas. Eur J Cancer. 2018; 92:40–7.

26. Ito Y, Miyauchi A, Hirokawa M, Yamamoto M, Oda H, Masuoka H, et al. Prognostic value of the 8th tumor-node-metastasis classification for follicular carcinoma and poorly differentiated carcinoma of the thyroid in Japan. Endocr J. 2018; 65:621–7.

27. Nunes da Silva T, Limbert E, Leite V. Poorly differentiated thyroid carcinoma patients with detectable thyroglobulin levels after initial treatment show an increase in mortality and disease recurrence. Eur Thyroid J. 2018; 7:313–8.

28. Bichoo RA, Mishra A, Kumari N, Krishnani N, Chand G, Agarwal G, et al. Poorly differentiated thyroid carcinoma and poorly differentiated area in differentiated thyroid carcinoma: is there any difference? Langenbecks Arch Surg. 2019; 404:45–53.

29. Akaishi J, Kondo T, Sugino K, Ogimi Y, Masaki C, Hames KY, et al. Prognostic impact of the Turin criteria in poorly differentiated thyroid carcinoma. World J Surg. 2019; 43:2235–44.

30. Wong KS, Lorch JH, Alexander EK, Marqusee E, Cho NL, Nehs MA, et al. Prognostic significance of extent of invasion in poorly differentiated thyroid carcinoma. Thyroid. 2019; 29:1255–61.

31. Kersting D, Seifert R, Kessler L, Herrmann K, Theurer S, Brandenburg T, et al. Predictive factors for RAI-refractory disease and short overall survival in PDTC. Cancers (Basel). 2021; 13:1728.

32. Panchangam RB, Puthenveetil P, Mayilvaganan S. Prognostic impact of focal poorly differentiated areas in follicular differentiated thyroid cancer: is it a distinct entity from poorly differentiated thyroid cancer? Indian J Surg Oncol. 2022; 13:157–63.
33. Xu B, David J, Dogan S, Landa I, Katabi N, Saliba M, et al. Primary high-grade non-anaplastic thyroid carcinoma: a retrospective study of 364 cases. Histopathology. 2022; 80:322–37.

34. Gubbiotti MA, Andrianus S, Sakhi R, Zhang Q, Montone K, Jalaly JB, et al. Does the presence of capsule influence prognosis in poorly differentiated thyroid carcinoma? Hum Pathol. 2023; 136:96–104.

35. Jeong SI, Kim W, Yu HW, Choi JY, Ahn CH, Moon JH, et al. Incidence and clinicopathological features of differentiated high-grade thyroid carcinomas: an institutional experience. Endocr Pathol. 2023; 34:287–97.

36. Patel KN, Shaha AR. Poorly differentiated and anaplastic thyroid cancer. Cancer Control. 2006; 13:119–28.

37. Ibrahimpasic T, Ghossein R, Carlson DL, Chernichenko N, Nixon I, Palmer FL, et al. Poorly differentiated thyroid carcinoma presenting with gross extrathyroidal extension: 1986-2009 Memorial Sloan-Kettering Cancer Center experience. Thyroid. 2013; 23:997–1002.

38. Thiagarajan S, Yousuf A, Shetty R, Dhar H, Mathur Y, Nair D, et al. Poorly differentiated thyroid carcinoma (PDTC) characteristics and the efficacy of radioactive iodine (RAI) therapy as an adjuvant treatment in a tertiary cancer care center. Eur Arch Otorhinolaryngol. 2020; 277:1807–14.

39. Bongiovanni M, Sadow PM, Faquin WC. Poorly differentiated thyroid carcinoma: a cytologic-histologic review. Adv Anat Pathol. 2009; 16:283–9.
40. Walczyk A, Kowalska A, Sygut J. The clinical course of poorly differentiated thyroid carcinoma (insular carcinoma): own observations. Endokrynol Pol. 2010; 61:467–73.
41. Ibrahimpasic T, Ghossein R, Carlson DL, Nixon I, Palmer FL, Shaha AR, et al. Outcomes in patients with poorly differentiated thyroid carcinoma. J Clin Endocrinol Metab. 2014; 99:1245–52.

42. Gerber TS, Schad A, Hartmann N, Springer E, Zechner U, Musholt TJ. Targeted next-generation sequencing of cancer genes in poorly differentiated thyroid cancer. Endocr Connect. 2018; 7:47–55.

43. Ibrahimpasic T, Ghossein R, Carlson DL, Nixon IJ, Palmer FL, Patel SG, et al. Undetectable thyroglobulin levels in poorly differentiated thyroid carcinoma patients free of macroscopic disease after initial treatment: are they useful? Ann Surg Oncol. 2015; 22:4193–7.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download