Abstract
In liver transplantation, the primary concern is to ensure an adequate future liver remnant (FLR) volume for the donor, while selecting a graft of sufficient size for the recipient. The living donor–resection and partial liver segment 2−3 transplantation with delayed total hepatectomy (LD−RAPID) procedure offers a potential solution to expand the donor pool for living donor liver transplantation (LDLT). We report the first case involving a cirrhotic patient with autoimmune hepatitis and hepatocellular carcinoma, who underwent left lobe LDLT using the LD−RAPID procedure. The living liver donor (LLD) underwent a laparoscopic left hepatectomy, including middle hepatic vein. The resection on the recipient side was an extended left hepatectomy, including the middle hepatic vein orifice and caudate lobe. At postoperative day 7, a computed tomography scan showed hypertrophy of the left graft from 320 g to 465 mL (i.e., a 45.3% increase in graft volume body weight ratio from 0.60% to 0.77%). After a 7-day interval, the diseased right lobe was removed in the second stage surgery. The LD−RAPID procedure using left lobe graft allows for the use of a small liver graft or small FLR volume in LLD in LDLT, which expands the donor pool to minimize the risk to LLD by enabling the donation of a smaller liver portion.
Living donor liver transplantation (LDLT) is the primary method of liver transplantation in regions with limited deceased donor (DD) availability. In liver transplantation, the primary concern is to ensure an adequate future liver remnant (FLR) volume for the donor, while selecting a graft of sufficient size for the recipient. Commonly, right lobe (RL) grafts are used for adult recipients to provide ample liver mass, despite potential risks for donors [1]. To enhance donor safety, increased use of left lobe (LL) grafts has been suggested. However, the graft-to-recipient weight ratio (GRWR) is crucial, and grafts with a GRWR < 0.8% pose risks like early allograft dysfunction and small-for-size syndrome (SFSS), potentially leading to graft failure and retransplantation [2,3].
Introduced in 2015, the resection and partial liver segment 2–3 transplantation with delayed total hepatectomy (RAPID) technique involves a staged approach. It includes transplanting the patient with a small auxiliary left liver graft, and ligating the right portal vein. The second stage involves performing residual hepatectomy when the transplanted graft has regenerated to a sufficient size. The living donor (LD)−RAPID procedure offers a potential solution to expand the donor pool for LDLT [4]. This approach aims to balance the need for an adequate liver mass for the recipient, while prioritizing donor safety.
Herein, we report the first case involving a cirrhotic patient with autoimmune hepatitis and hepatocellular carcinoma (HCC), who underwent LL LDLT using the LD−RAPID procedure.
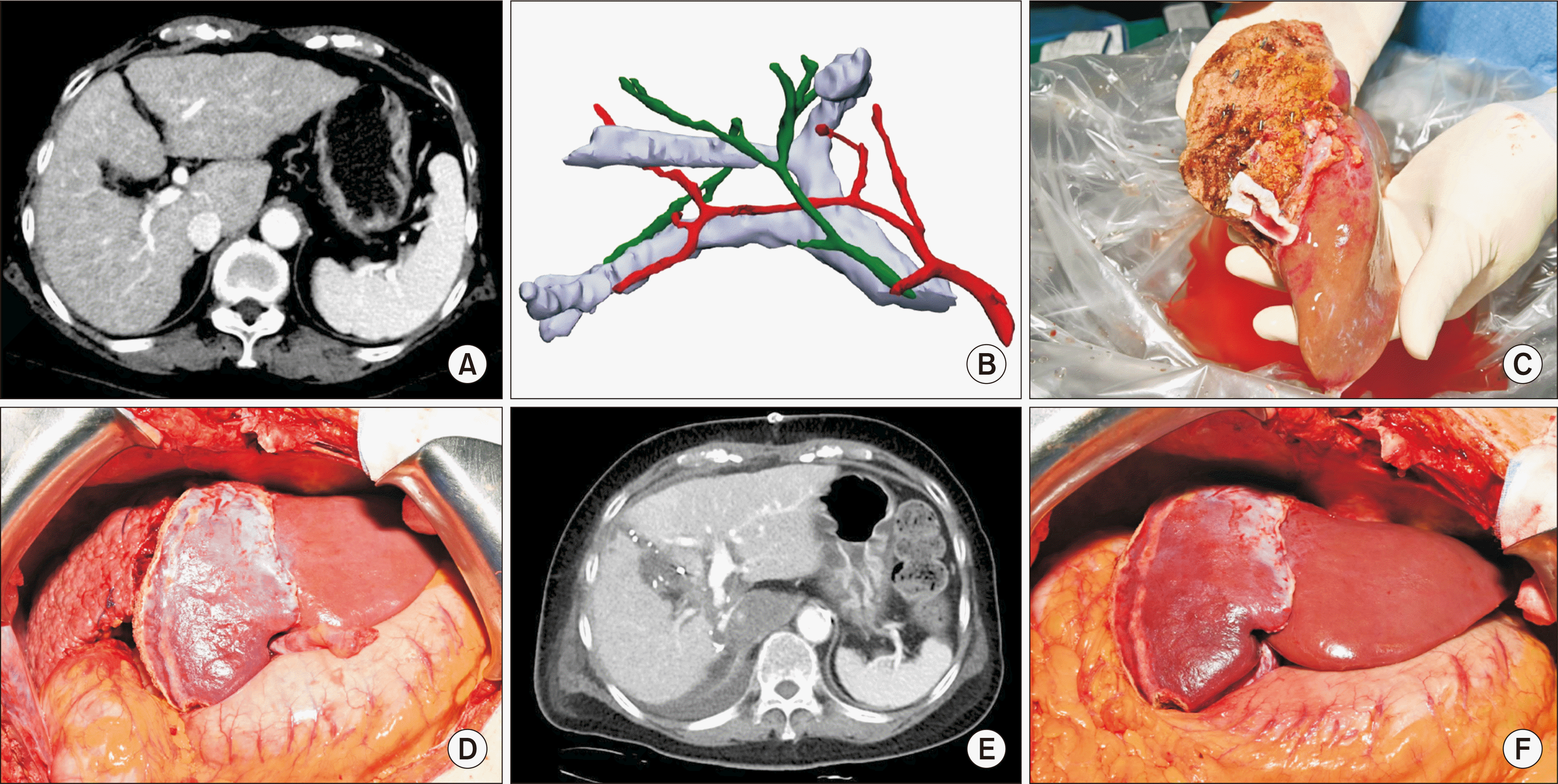
The patient was a 72-year-old female, of 54 kg body weight and 157 cm height, who presented with HCC in the setting of cirrhosis due to autoimmune hepatitis. The HCC in segment 7 was managed using radiofrequency ablation (RFA), and the imaging studies confirmed the presence of viable HCC. Furthermore, the patient’s liver exhibited cirrhotic changes, as depicted in Fig. 1A. Splenomegaly was observed, and the laboratory results revealed a total bilirubin level of 1.1 mg/dL, and a model for end-stage liver disease (MELD) score of 9. In terms of potential liver transplantation, the only suitable donor within the family was the patient’s 38-year-old son. A three-dimensional volumetric analysis of the son’s liver indicated a future remnant liver volume of 26% and mild to moderate fatty changes on preoperative imaging, as shown in Fig. 1B. Consequently, the LL was selected for liver procurement to prioritize the safety of the donor. The estimated GRWR was calculated to be 0.69, with a graft volume of 414 mL, which was deemed adequate for the recipient in a single-stage operation. The son of the patient exhibited a weight of 89.6 kg during the period of assessment for being a potential donor, subsequently undergoing a decline to 83 kg within a span of two months.
Although the liver graft in the living liver donor (LLD) exhibited 5% macrosteatosis and 5% microsteatosis, the left graft appeared smaller than anticipated. A decision was made to employ the RAPID procedure in the recipient during laparoscopic donor hepatectomy. The LLD underwent a laparoscopic left hepatectomy, which included the middle hepatic vein (Supplementary Material 1). The weight of the LL graft was measured to be 320 g, with an actual GRWR of 0.6%. The hepatic vein orifice was expanded utilizing a cadaveric iliac vein fence in the bench procedure (Fig. 1C).
The resection performed on the recipient side entailed an extended left hepatectomy, which included the orifice of the middle hepatic vein, as well as the caudate lobe. In the explant left liver, only cirrhosis was present without HCC. The graft of the LL was implanted through an anastomosis that connected the middle-left hepatic vein of the graft to the unified middle-left hepatic vein stump. Furthermore, the left portal vein and left hepatic artery were anastomosed to the recipient’s left portal vein and left hepatic artery, respectively. Subsequently, the right portal vein was severed, and both ends were sutured together after blood perfusion. Biliary reconstruction was successfully accomplished through the implementation of a duct-to-duct anastomosis, connecting the left bile duct in the donor to the left bile duct in the recipient (Supplementary Material 2). The total duration of the recipient’s surgical procedure lasted 420 minutes, with a recorded blood loss of 600 mL. Additionally, she was administered 2 pints of red blood cells during the operation (Fig. 1D).
Immunosuppressive therapy entailed the administration of basiliximab for induction therapy and the use of steroids, tacrolimus, and mycophenolate mofetil for maintenance therapy, following our established protocol after liver transplantation. On the seventh day postsurgery, a computed tomography scan revealed a notable increase in the volume of the left graft, from 320 g to 465 mL, indicating a 45.3% augmentation in the graft volume-to-body weight ratio, from 0.60% to 0.77% (Fig. 1E).
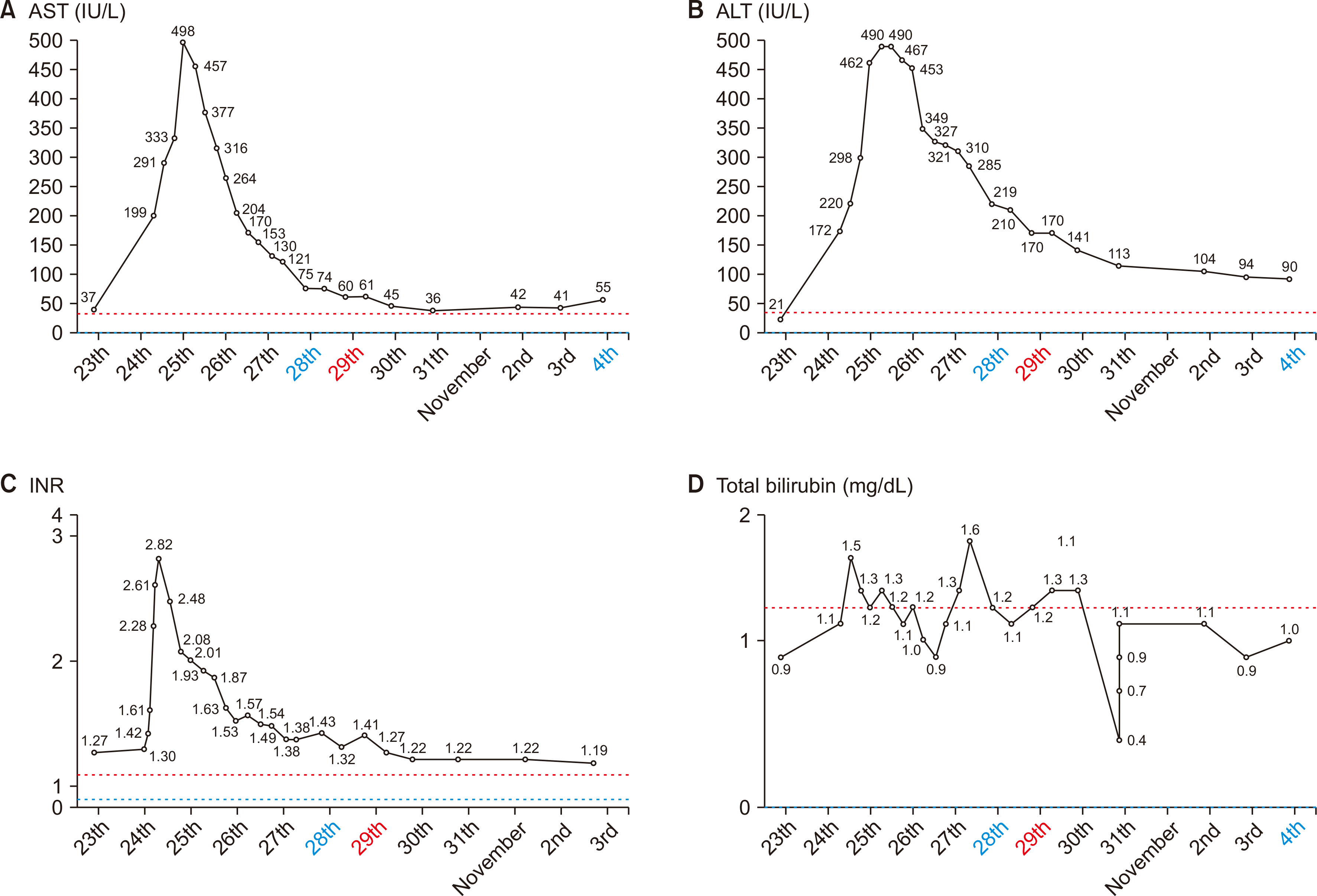
The recipient experienced no complications following the first stage of the operation. Fig. 2 depicts the postoperative progression of laboratory values, including alanine transaminase, international normalized ratio, and bilirubin.
After a duration of 7 days, the affected RL was extracted during the subsequent surgical procedure (Fig. 1F). Solitary HCC was found in the explant right liver, and the tumor size was 3.4 cm, but the viable HCC was 1 cm. There was no microvascular invasion. After the operation, the patient experienced a smooth recovery, and was released from the medical facility on postoperative day 18. The transplanted graft exhibited consistent favorable performance and adequate blood circulation. Additionally, the postoperative progress of the LLD was entirely free from complications, leading to the patient being discharged from the hospital on postoperative day 6. At the time of writing (late February 2024), there have been no instances of medical or psychological complications.
The presented case report marks the inaugural success of the LD−RAPID procedure using a LL graft (GRWR, 0.6%) for LDLT in an adult patient afflicted with cirrhotic liver disease, autoimmune hepatitis, and HCC. Despite the persistent challenge of organ scarcity from DDs, the LD−RAPID procedure offers a promising avenue to substantially broaden the donor pool while concurrently diminishing risks to donors, thereby facilitating timely access to potentially curative treatments.
The LD−RAPID technique, recently introduced by Königsrainer et al. [5], has emerged as a viable and secure alternative to the original DD−RAPID procedure. Patients with decompensated liver cirrhosis, characterized by low scores on the MELD and limited options from DD grafts, stand to benefit from the LD−RAPID procedure. Notably, this approach also may allow for the inclusion of donors with small FLRs, who might otherwise be disqualified for RL donation, thereby further augmenting the donor pool.
Transplanting smaller liver grafts in adult recipients introduces specific physiological and surgical challenges. The diminutive liver volume necessitates a well-functioning liver remnant in the recipient, acting as a safeguard until the transplanted graft achieves sufficient size and functional capacity. Consequently, recipient hepatectomy is performed in two stages. Additionally, there is a significant concern posed by the susceptibility of a very small graft to the SFSS, characterized by portal hyperperfusion and elevated portal pressures, potentially leading to liver dysfunction or failure [2].
Portocaval shunt should be performed in patients with portal hypertension [4], but in our case, ligation was performed without measuring the portal vein pressure. This is a limitation of our case; hence from the next case, we must decide whether to perform a portocaval shunt based on portal pressure. In addition, oncological outcomes are a critical consideration, particularly for patients with HCC undergoing this technique. In this case, prior treatment of HCC with RFA resulted in a small viable tumor in pretransplant images, and 90% tumor necrosis in the explant pathology. The deliberate 7-day interval between the first and second surgeries aims to minimize HCC recurrence. This approach holds the potential to offer timely access to LDLT for HCC patients on the waiting list, particularly those within the Milan criteria.
In conclusion, the LD−RAPID procedure using LL graft represents a viable option for patients with cirrhosis and HCC. This innovative approach allows for the use of a small GRWR graft or small FLR volume in LLD in LDLT, which expands the donor pool while offering timely curative treatment to patients with decompensated cirrhosis and relatively low MELD scores. Importantly, by enabling the donation of a smaller liver portion, it minimizes the risk to LLDs.
Supplementary data related to this article can be found at https://youtu.be/wAd4mlEK-k4 and https://youtu.be/J91vUmFbECs.
References
1. Choi JY, Kim JH, Kim JM, Kim HJ, Ahn HS, Joh JW. 2022; Outcomes of living liver donors are worse than those of matched healthy controls. J Hepatol. 76:628–638. DOI: 10.1016/j.jhep.2021.10.031. PMID: 34785324.
2. Kow AWC, Liu J, Patel MS, De Martin E, Reddy MS, Soejima Y, et al. 2023; Post living donor liver transplantation small-for-size syndrome: definitions, timelines, biochemical, and clinical factors for diagnosis: guidelines from the ILTS-iLDLT-LTSI consensus conference. Transplantation. 107:2226–2237. DOI: 10.1097/TP.0000000000004770. PMID: 37749812.
3. Kirchner VA, Shankar S, Victor DW 3rd, Tanaka T, Goldaracena N, Troisi RI, et al. 2023; Management of established small-for-size syndrome in post living donor liver transplantation: medical, radiological, and surgical interventions: guidelines from the ILTS-iLDLT-LTSI consensus conference. Transplantation. 107:2238–2246. DOI: 10.1097/TP.0000000000004771. PMID: 37749813.
4. Balci D, Kirimker EO, Kologlu MB, Ustuner E, Erkoc SK, Cinar G, et al. 2022; Left lobe living donor liver transplantation using rapid procedure in a cirrhotic patient with portal vein thrombosis. Ann Surg. 275:e538–e539. DOI: 10.1097/SLA.0000000000005107. PMID: 34334650.
5. Königsrainer A, Templin S, Capobianco I, Königsrainer I, Bitzer M, Zender L, et al. 2019; Paradigm shift in the management of irresectable colorectal liver metastases: living donor auxiliary partial orthotopic liver transplantation in combination with two-stage hepatectomy (LD-RAPID). Ann Surg. 270:327–332. DOI: 10.1097/SLA.0000000000002861. PMID: 29916882.
Fig. 1
(A) Preoperative CT image in recipient. (B) Illustration of living liver donor anatomy. (C) Left liver graft after bench procedure. (D) Operative field after first operation on the recipient. (E) CT image at postoperative day 7 after first operation on the recipient. (F) Operative field after second operation on the recipient. CT, computed tomography.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download