Abstract
Backgrounds/Aims
The published data had contradictory information on the role of adjuvant therapy on resected periampullary carcinomas (PACA). The study was performed to evaluate the survival benefit of adjuvant treatment.
Methods
This was a propensity score matched case-control study from a prospectively maintained database from 2004–2019. The study included patients with nonpancreatic PACA who underwent curative resection. The patients (cases) who received adjuvant chemotherapy were compared with patients (controls) who were observed alone after surgery.
Results
Of 510 patients with PACA, 230 patients (cases = 107, controls = 123) formed the unmatched study cohort. After propensity score matching, 140 patients (cases = 70, controls = 70) formed the matched study cohort. The median overall survival (OS) was similar in cases than controls in the unmatched population but doubled non-significantly in cases after matching (unmatched population, 54 months vs. 54 months, p-value = 0.624; matched population, 71 months vs. 36 months, p-value = 0.087). However, the median recurrence-free survival (RFS) was non significantly higher in the control group (unmatched population, 59 months vs. 38 months, p-value = 0.195; matched population, 53 months vs. 40 months, p-value = 0.797). In cox regression analysis, age < 60 years, advanced T stage, and presence of perineural invasion were independent factors for worse RFS, while tumor recurrence was an independent factor for poor OS.
The periampullary region has peculiar histological features intersecting three different epithelial linings (intestinal, biliary, and pancreatic) determining the respective prognoses and treatment outcomes of tumors arising here. On account of their anatomical location, they appear early with features of obstructive jaundice, resulting in early diagnosis at an early stage. Till recently, they had been clubbed together with pancreatic cancers and treated similarly, with the primary modality being pancreatoduodenectomy with or without pylorus preservation; consequently, the reported survival rates vary vastly. The reported resection rates for periampullary carcinomas (PACA) are high, ranging from 76.5% to 89.4%, compared to 10% for pancreatic cancers [1-6]. Despite radical surgery, recurrences plague these tumors both early and late in the course. Segregation of pancreatic cancers from PACA has resulted in better understanding and management; however, the treatment strategies continue to be inspired by trials covering adjacent organs i.e., pancreas or colon or biliary tract cancers perse [7,8]. The rarity of the disease precludes randomized trials to assess the utility of adjuvant treatment in these cancers.
Akin to pancreatic cancers, several attempts have been made to control these recurrences using adjuvant treatments with conflicting results [6,9-11]. Due to the lack of evidence-based management guidelines in these cases, the use of adjuvant therapy is not uniform. Consequently, published data yields contradictory information regarding the utility of adjuvant treatments.
As a tertiary care center of the large footfall of periampullary cases treated with radical surgery with and without adjuvant chemotherapy, we conducted a propensity score-matched case-control study to assess whether any survival benefit was associated with adjuvant chemotherapy compared with observation alone in patients with resected periampullary adenocarcinomas.
This case-control study was conducted in the Department of Radiotherapy, Maulana Azad Medical College and Department of Gastrointestinal Surgery, GB Pant Hospital. It was reported according to STROBE guidelines [12]. A prospectively maintained database reviewed all patients diagnosed with PACA treated by a single unit from 2004 to 2019. The institutional review board approved the study (IEC/MAMC/89/01/2022/No.66). The informed patient consent was obtained.
All Patients, including nonpancreatic PACA, underwent curative resection during the study period. Patients having either unresectable locally advanced disease or distant metastases, patients with postoperative mortality, i.e., within 90 days of surgery, were excluded from the analysis. These excluded periampullary tumors with histology other than adenocarcinoma or pancreatic involvement on histopathological examination, as well as patients who started chemotherapy after 90 days of surgery to avoid including patients who probably received chemotherapy for a recurrence of the disease.
The retrieved data reflects demography, mode of treatment; site of origin (radiological and pathological), overall survival (OS), and pattern of recurrences. Subtyping of the disease in terms of intestinal, pancreatobiliary and ambiguous type was only uniformly available for some cases as this became standard practice at a later time. Recurrence/relapse were taken as an event from the date of first diagnosis (radiological/pathological). Recurrences were grouped as locoregional when confined to the tumor bed and the regional lymph nodes, distant when the disease spread to non-regional nodes, liver, peritoneum, other distant visceral organs, or both when detected synchronously.
The primary outcome was OS, the interval between the surgery date and death due to any cause. The secondary outcome was recurrence-free survival (RFS), the interval from surgery to the time of documented recurrence (radiologically/pathologically) or date of death secondary to non-malignant causes or the last visit without recurrence.
The last follow up (physical visit/telephonic consultation) was taken on 31st May 2020. For analysis, we deemed any patient who had not been traceable telephonically or had not reported for a physical outpatient department (OPD) visit for more than two years from the last physical visit as ‘lost to follow up.’
Patients diagnosed with resectable PACA were offered pancreaticoduodenectomy with or without pylorus preservation. When indicated, patients underwent preoperative biliary drainage. Adjuvant treatment was not a standard procedure. The treating oncologist assessed the patient’s presentation in the oncology OPD 6–8 weeks postoperatively. We started adjuvant treatment after ensuring complete recovery from post-surgical effects, i.e., 6–10 weeks after surgery. The adjuvant treatment arm (cases) included 6 cycles of chemotherapy. Adjuvant chemotherapy was given at the discretion of the physician, primarily for advanced T stage, node positivity, poor differentiation and non-R0 resection while considering the patient’s fitness and age and site of disease. Adjuvant chemotherapy was quite varied, keeping with the evolving evidence in literature over a long, extended period. Thus, regimens used were either a combination of cisplatin 80 mg/m2 D1 + 5 fluorouracil 800 mg/m2 D1-3 or gemcitabine 1,000 mg/m2 D1, D8 + cisplatin 30 mg/m2 D1, D8 or capecitabine 1 gm/m2 D1-14 + oxaliplatin 130 mg/m2 D1 as well as single agent gemcitabine or capecitabine in selected cases who were deemed unfit for multiagent chemotherapy. Cycles were delivered at intervals of 3–4 weeks each for a total number of 6 cycles. Patients not included in the adjuvant treatment arm (control group) were kept under regular surveillance.
The patients were followed with abdominal imaging (contrast enhanced computed tomography) every 6 months for the first two years and then annually for the subsequent 2 years. After that, imaging was performed when indicated based on the patient’s symptoms. Chest X-rays were performed annually for the initial two years and repeated earlier if symptomatically indicated, while bone scans were performed only in cases of high index of suspicion.
SPSS Version 28.0 (IBM Corp.) was used for statistical analysis. Propensity score matching (1:1, nearest neighbor matching) was done using covariates as T stage, N stage, age, Eastern Cooperative Oncology Group performance status, sex. Categorical data were compared using chi-square or Fisher’s exact test. Continuous data were compared by either the Student’s t-test or Mann–Whitney U test. Kaplan–Meier (KM) survival curves were used for survival analysis and were compared with a log-rank test. The Cox proportion model analyzed univariate and multivariate analyses of various risk factors for OS and RFS. A Forrest plot was constructed using hazard ratio (HR) obtained for various parameters. The KM survival curves and Forrest plot were constructed using Medcalc statistical software 20.014 (Medcalc Software Ltd.; https://www.medcalc.org; 2021). A p-value < 0.05 was considered statistically significant. The statistical tests were two-sided, with 5% defined as the significance level.
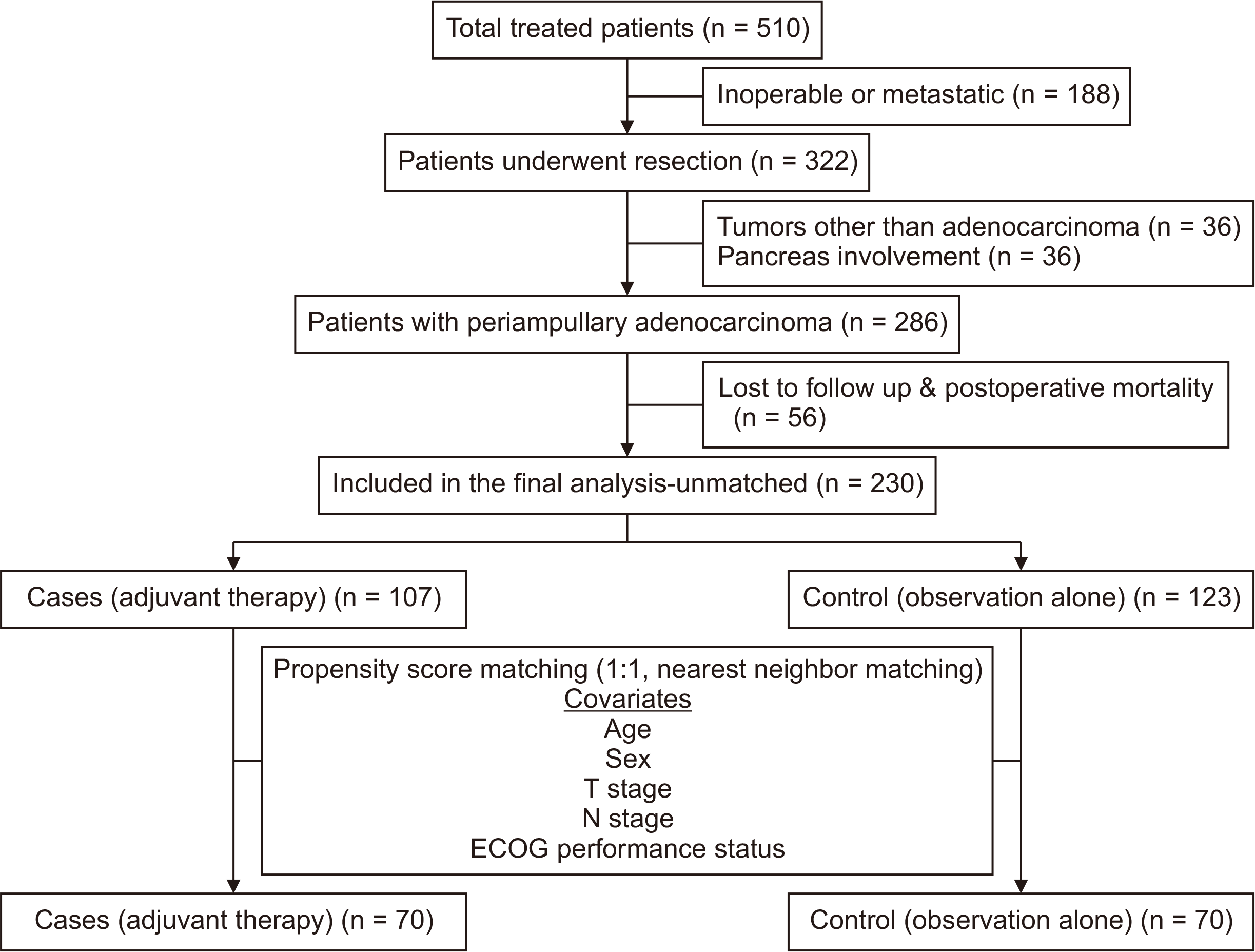
A total of 510 patients were admitted with a diagnosis of PACA. After baseline investigative evaluation, 188 patients were deemed inoperable either based on locally advanced stage or the presence of distant metastases. A total of 322 patients underwent surgery, and after a review of the histopathology reports, 36 were excluded because of either pancreatic involvement or non-adenocarcinoma diagnosis. Of the remaining 286 patients diagnosed with adenocarcinoma, 56 patients were excluded due to postoperative mortality (n = 5), or lost to follow-up (n = 51), leaving 230 patients (cases = 107, controls = 123) for unmatched analysis. On propensity matching, 140 patients (cases = 70, controls = 70) were included (Fig. 1).
The mean age of the control group was significantly higher than cases in the matched and unmatched population. Ampulla was the most common site for tumors (unmatched population, 71.7%; matched population, 70.7%). Although tumors with advanced T stage, N stage, and overall stage were significantly more in the adjuvant therapy group in the unmatched population, there was no significant difference in the T stage, N stage, and overall stage between the groups after matching. The majority of study population received gemcitabine plus cisplatin (Table 1).
The overall recurrence in the unmatched and matched populations was 43.9% and 42.8%, respectively (Table 2). The proportion of patients with recurrences was similar between the case and control groups. However, distant failures were commoner in the control group (unmatched, 30.1% vs. 21.5%; matched, 34.3% vs. 20.0%). The incidence of death was higher in the control group than cases (unmatched population, 48.8% vs. 37.4%, p = 0.082; matched population, 57.1% vs. 31.4%, p = 0.002).
The median OS was similar in cases than controls in the unmatched population but was non-significantly doubled in cases after matching (unmatched population, 54 months vs. 54 months, p = 0.624; matched population, 71 months vs. 36 months, p = 0.087) (Table 2, Fig. 2). However, the median RFS was non-significantly higher in the control group (unmatched population, 59 months vs. 38 months, p = 0.195; matched population, 53 months vs. 40 months, p = 0.797) (Fig. 3).
The median OS and RFS among ampullary, duodenal, and biliary tumors were significantly different from each other (OS, 64 months vs. 33 months vs. 28 months, p < 0.001; RFS, 62 months vs. 30 months vs. 34 months, p = 0.011). The median OS and RFS were significantly better in the early T stage (T1, T2) than in the late T stage (T3, T4) (OS, not achieved vs. 39 months, p = 0.001; RFS, not achieved vs. 30 months, p < 0.001). The median OS and RFS were significantly better in node-negative than node-positive tumors (OS, 65 months vs. 39 months, p < 0.001; RFS, not achieved vs. 30 months, p < 0.001).
Univariate analysis of various factors affecting OS revealed that non-ampullary tumors, advanced T stage, N stage, presence of perineural invasion (PNI), lymph node ratio (LNR) > 0.1, and tumor recurrence were significantly associated with worse survival. On cox regression analysis, tumor recurrence was an independent predictor of poor OS (Table 3). On univariate analysis of various factors for RFS, age < 60 years, non-ampullary tumors, advanced T stage, N stage, presence of PNI, and LNR > 0.1 were significantly associated with worse survival. However, on cox regression analysis, age < 60 years, advanced T stage, and presence of PNI were independent factors for poor RFS (Table 4). On stratification analysis, patients with well-differentiated or moderately differentiated tumors had significant OS benefits with adjuvant chemotherapy (HR, 0.41; 95% confidence interval, 0.21–0.78; p < 0.001) (Fig. 4).
To our knowledge, this is one of India’s largest single centre experiences from a teaching training institute, including prospectively maintained data of 230 PACA. Despite radiological and pathological evaluation differentiating between ampullary, duodenal and bile duct cancers may not be precise [13] hence, we studied them together as nonpancreatic PACA without site wise stratification. Treatment outcomes of the nonpancreatic PACA treated over the last 15 years at our centre were evaluated using propensity score analysis to assess the effectiveness of adjuvant chemotherapy in completely resected PACA. In the unmatched cohort, the median survival of 54 months was reported for both cases and controls. However, after propensity score matching, the median survival reported was 71 months in the adjuvant treatment arm compared to 36 months in the control arm. Though the T stage, N stage, overall TNM stage, and LNRs differed systematically between groups, the survival benefit was high. The statistically non-significant effect observed in the matched population likely represented an actual effect, as the baseline tumor factors were distributed evenly between the groups after matching.
This study showed an overall recurrence rate of 44%, akin to that reported in the literature, of 14% to 45% [14]. Distant metastasis was the predominant mode of recurrence, two-thirds of all recurrences occurring within two years of surgery. Although distant metastases outnumbered locoregional recurrences in both arms, the distant recurrences were proportionately higher in the observation arm, again pointing towards need for effective systemic treatment. Recurrences were reported as late as 72 months after surgery, underlining the need for prolonged follow-up [15]. Liver was the most common site of distant recurrence followed by peritoneum, bone, and lung, similar to that reported in the literature [16].
The survival benefit of adjuvant chemotherapy in the treatment of pancreatic cancer has been extrapolated to PACA. However, PACA are distinct from pancreatic carcinoma due to their relatively earlier presentation with obstructive jaundice. This facilitates early diagnosis and surgical resection of a relatively localized tumor that has yet to invade the lymphovascular spaces or neural tracts. Hence, surgical outcomes are better for PACA than pancreatic carcinomas. The usage of intravenous and intra-arterial chemotherapy with and without radiotherapy is reported [17-20]. However, the major drawback of these studies has been the clubbing together of both periampullary and pancreatic cancers.
The largest randomized trial assessing the role of adjuvant treatment in PACA was the ESPAC-3 trial, which compared the use of gemcitabine or fluorouracil chemotherapy with observation. Although it failed to demonstrate survival benefits with adjuvant chemotherapy compared to observation in its primary analysis of 434 patients, a subsequent subset analysis of 304 ampullary cases showed non-significantly improved OS with adjuvant chemotherapy. As a subset analysis, the results could not be interpreted as conclusive evidence favouring adjuvant chemotherapy [6].
The role of additional local therapy in adjuvant radiotherapy with or without chemotherapy has also been explored without much success, as seen in the EORTC 40,891 trial with 93 PACA [21].
A recent meta-analysis of adjuvant therapy for periampullary adenocarcinoma following curative resection found no survival benefit with adjuvant chemotherapy or chemoradiotherapy. One of the limitations of the meta-analysis was that three out of 14 studies included in the analysis also included patients with carcinoma-in-situ. Additionally, the need for more adequate numbers in the various groups under study forgoes the demonstration of any real benefit of adjuvant therapy [10].
Despite the negative randomized controlled trials and meta-analysis, there is substantial published data favouring survival benefits with adjuvant treatment in these cases. The National Cancer Database analysis of the USA recently showed that adjuvant chemotherapy was associated with survival benefit in patients with resected ampullary carcinoma, including node-negative and margin-negative patients [22]. A multicenter study from Korea reported that non-adjuvant group had more significant OS and RFS benefits than the adjuvant group in patients in the early stages (T1N0, T2N0). However, Node positive and T3 and T4 stage patients had a non-statistically significant survival with adjuvant therapy [23].
There are certain limitations of the study. The retrospective nature of the study is foremost. Secondly, the heterogenous adjuvant chemotherapy schedules followed in the study posed difficulty in interpreting results. Additionally, there was a lack of information regarding the completion of chemotherapy and the associated toxicities that precludes the actual impact of adjuvant chemotherapy, more so in early-stage groups. Thirdly, there was a significant loss in follow-up (17%) that may have impacted the outcomes. Longitudinal follow-up in India in such an “out of trial” set is plagued with the problem of irregular follow-up of patients [24]. Considering the worst-case scenario, all lost-to-follow-up patients were eliminated from the analysis, so the actual impact on outcome may be different. Although the propensity score matching attempted to reduce the selection bias, it cannot be eliminated. Recurrence is the alone, independent factor for the OS on Cox regression analysis, which might indicate the larger sampling variability of the study population.
References
1. Nakase A, Matsumoto Y, Uchida K, Honjo I. 1977; Surgical treatment of cancer of the pancreas and the periampullary region: cumulative results in 57 institutions in Japan. Ann Surg. 185:52–57. DOI: 10.1097/00000658-197701000-00008. PMID: 831636. PMCID: PMC1396263.
2. Todoroki T, Koike N, Morishita Y, Kawamoto T, Ohkohchi N, Shoda J, et al. 2003; Patterns and predictors of failure after curative resections of carcinoma of the ampulla of Vater. Ann Surg Oncol. 10:1176–1183. DOI: 10.1245/ASO.2003.07.512. PMID: 14654474.
3. Howe JR, Klimstra DS, Moccia RD, Conlon KC, Brennan MF. 1998; Factors predictive of survival in ampullary carcinoma. Ann Surg. 228:87–94. DOI: 10.1097/00000658-199807000-00013. PMID: 9671071. PMCID: PMC1191432.
4. Choi SB, Kim WB, Song TJ, Suh SO, Kim YC, Choi SY. 2011; Surgical outcomes and prognostic factors for ampulla of Vater cancer. Scand J Surg. 100:92–98. DOI: 10.1177/145749691110000205. PMID: 21737384.
5. Riall TS, Cameron JL, Lillemoe KD, Winter JM, Campbell KA, Hruban RH, et al. 2006; Resected periampullary adenocarcinoma: 5-year survivors and their 6- to 10-year follow-up. Surgery. 140:764–772. DOI: 10.1016/j.surg.2006.04.006. PMID: 17084719.
6. Neoptolemos JP, Moore MJ, Cox TF, Valle JW, Palmer DH, McDonald AC, et al. 2012; Effect of adjuvant chemotherapy with fluorouracil plus folinic acid or gemcitabine vs observation on survival in patients with resected periampullary adenocarcinoma: the ESPAC-3 periampullary cancer randomized trial. JAMA. 308:147–156. DOI: 10.1001/jama.2012.7352. PMID: 22782416.
7. Duan Z, Zhang Y, Tang Y, Gao R, Bao J, Liang B. 2022; Adjuvant therapy for periampullary carcinoma and the significance of histopathological typing: a systematic review. Transl Oncol. 20:101414. DOI: 10.1016/j.tranon.2022.101414. PMID: 35397420. PMCID: PMC9006738.
8. de Jong EJM, Mommers I, Fariña Sarasqueta A, van der Geest LG, Heij L, de Hingh IHJT, et al. 2022; Adjuvant and first-line palliative chemotherapy regimens in patients diagnosed with periampullary cancer: a short report from a nationwide registry. Acta Oncol. 61:591–596. DOI: 10.1080/0284186X.2022.2053199. PMID: 35382678.
9. Balachandran P, Sikora SS, Kapoor S, Krishnani N, Kumar A, Saxena R, et al. 2006; Long-term survival and recurrence patterns in ampullary cancer. Pancreas. 32:390–395. DOI: 10.1097/01.mpa.0000220864.80034.63. PMID: 16670621.
10. Acharya A, Markar SR, Sodergren MH, Malietzis G, Darzi A, Athanasiou T, et al. 2017; Meta-analysis of adjuvant therapy following curative surgery for periampullary adenocarcinoma. Br J Surg. 104:814–822. DOI: 10.1002/bjs.10563. PMID: 28518410.
11. Bhandare MS, Mondal A, Chaudhari V, Bal M, Yadav S, Ramaswamy A, et al. 2021; Factors influencing local and distant recurrence following resection of periampullary cancer. Br J Surg. 108:427–434. DOI: 10.1093/bjs/znaa143. PMID: 33723577.
12. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. STROBE Initiative. 2008; The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 61:344–349. DOI: 10.1016/j.jclinepi.2007.11.008. PMID: 18313558.
13. Ostwal V, Harris C, Sirohi B, Goel M, Bal M, Kannan S, et al. 2017; Role of adjuvant chemotherapy in T2N0M0 periampullary cancers. Asia Pac J Clin Oncol. 13:e298–e303. DOI: 10.1111/ajco.12612.
14. Sunil BJ, Seshadri RA, Gouthaman S, Ranganathan R. 2017; Long-term outcomes and prognostic factors in periampullary carcinoma. J Gastrointest Cancer. 48:13–19. DOI: 10.1007/s12029-016-9863-z. PMID: 27484980.
15. Park HM, Park SJ, Han SS, Hong SK, Hong EK, Kim SW. 2019; Very early recurrence following pancreaticoduodenectomy in patients with ampullary cancer. Medicine (Baltimore). 98:e17711. DOI: 10.1097/MD.0000000000017711. PMID: 31689805. PMCID: PMC6946574.
16. Kim H, Kwon W, Kim JR, Byun Y, Jang JY, Kim SW. 2019; Recurrence patterns after pancreaticoduodenectomy for ampullary cancer. J Hepato-Biliary-Pancreat Sci. 26:179–186. DOI: 10.1002/jhbp.618. PMID: 30849209.
17. Bhatia S, Miller RC, Haddock MG, Donohue JH, Krishnan S. 2006; Adjuvant therapy for ampullary carcinomas: the Mayo Clinic experience. Int J Radiat Oncol Biol Phys. 66:514–519. DOI: 10.1016/j.ijrobp.2006.04.018. PMID: 16863684.
18. Ghosn M, Kourie HR, El Rassy E, Haddad FG, Hanna C, El Karak F, et al. 2016; Where does chemotherapy stands in the treatment of ampullary carcinoma? A review of literature. World J Gastrointest Oncol. 8:745–750. DOI: 10.4251/wjgo.v8.i10.745. PMID: 27795814. PMCID: PMC5064052.
19. Klinkenbijl JH, Jeekel J, Sahmoud T, van Pel R, Couvreur ML, Veenhof CH, et al. 1999; Adjuvant Radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region: phase III trial of the EORTC gastrointestinal tract cancer cooperative group. Ann Surg. 230:776–782. DOI: 10.1097/00000658-199912000-00006. PMID: 10615932. PMCID: PMC1420941.
20. Morak MJM, van der Gaast A, Incrocci L, van Dekken H, Hermans JJ, Jeekel J, et al. 2008; Adjuvant intra-arterial chemotherapy and radiotherapy versus surgery alone in resectable pancreatic and periampullary cancer: a prospective randomized controlled trial. Ann Surg. 248:1031–1041. DOI: 10.1097/SLA.0b013e318190c53e. PMID: 19092348.
21. Smeenk HG, van Eijck CHJ, Hop WC, Erdmann J, Tran KC, Debois M, et al. 2007; Long-term survival and metastatic pattern of pancreatic and periampullary cancer after adjuvant chemoradiation or observation: long-term results of EORTC trial 40891. Ann Surg. 246:734–740. DOI: 10.1097/SLA.0b013e318156eef3. PMID: 17968163.
22. Kamarajah SK, Bednar F, Cho CS, Nathan H. 2021; Survival benefit of adjuvant chemotherapy after pancreatoduodenectomy for ampullary adenocarcinoma: a propensity-Matched National Cancer Database (NCDB) analysis. J Gastrointest Surg. 25:1805–1814. DOI: 10.1007/s11605-020-04879-x. PMID: 33230687. PMCID: PMC8275534.
23. Kim HS, Jang JY, Yoon YS, Park SJ, Kwon W, Kim SW, et al. 2020; Does adjuvant treatment improve prognosis after curative resection of ampulla of Vater carcinoma? A multicenter retrospective study. J Hepatobiliary Pancreat Sci. 27:721–730. DOI: 10.1002/jhbp.801. PMID: 32652820.
24. Swaminathan R, Rama R, Shanta V. 2008; Lack of active follow-up of cancer patients in Chennai, India: implications for population-based survival estimates. Bull World Health Organ. 86:509–515. DOI: 10.2471/BLT.07.046979. PMID: 18670662. PMCID: PMC2647482.
Fig. 2
(A) Overall survival between two treatment groups before matching. (B) Overall survival between two treatment groups after matching. HR, hazard ratio; CI, confidence interval.
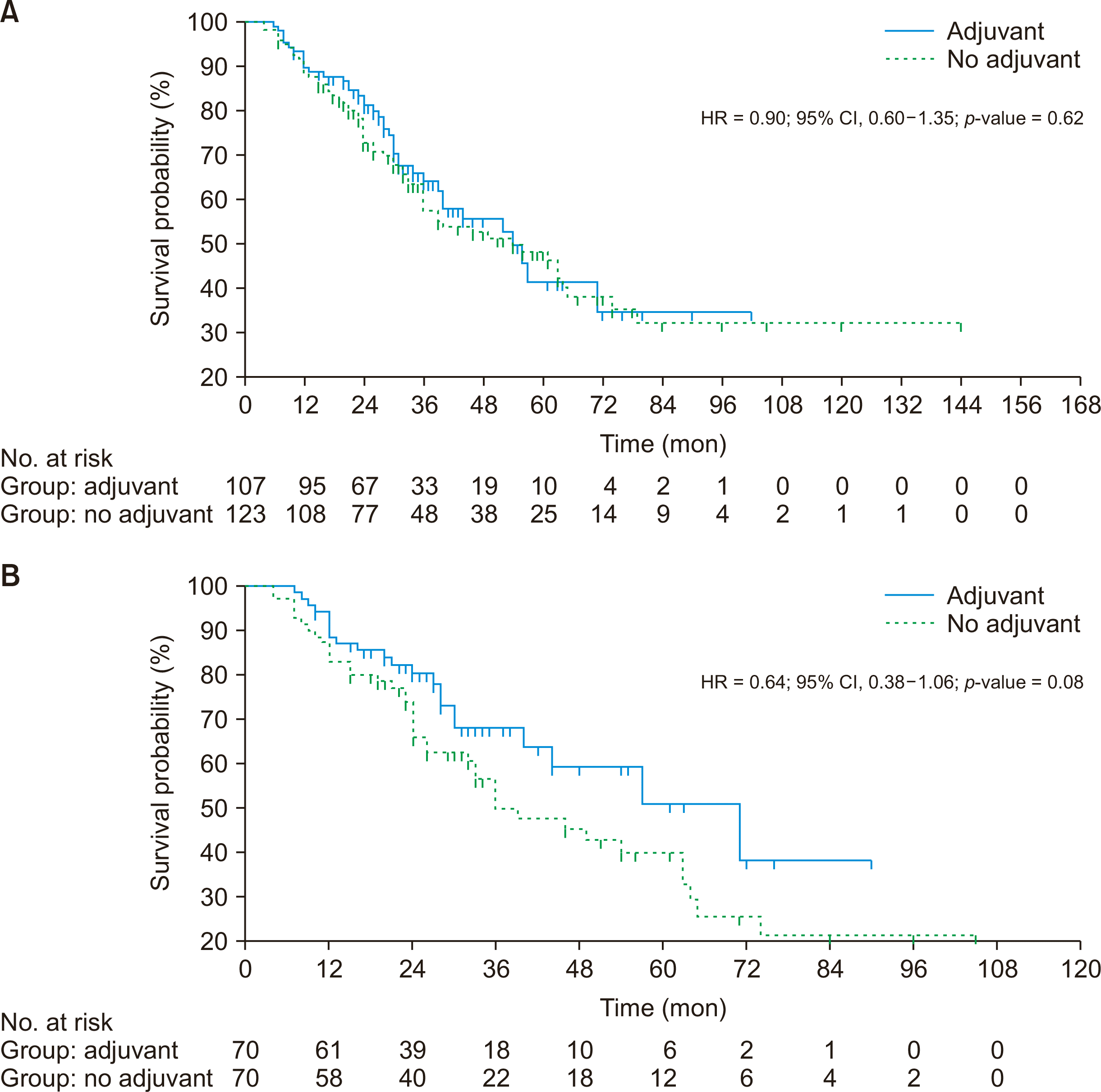
Fig. 3
(A) Recurrence-free survival between two treatment groups before matching. (B) Recurrence-free survival between two treatment groups after matching. HR, hazard ratio; CI, confidence interval.
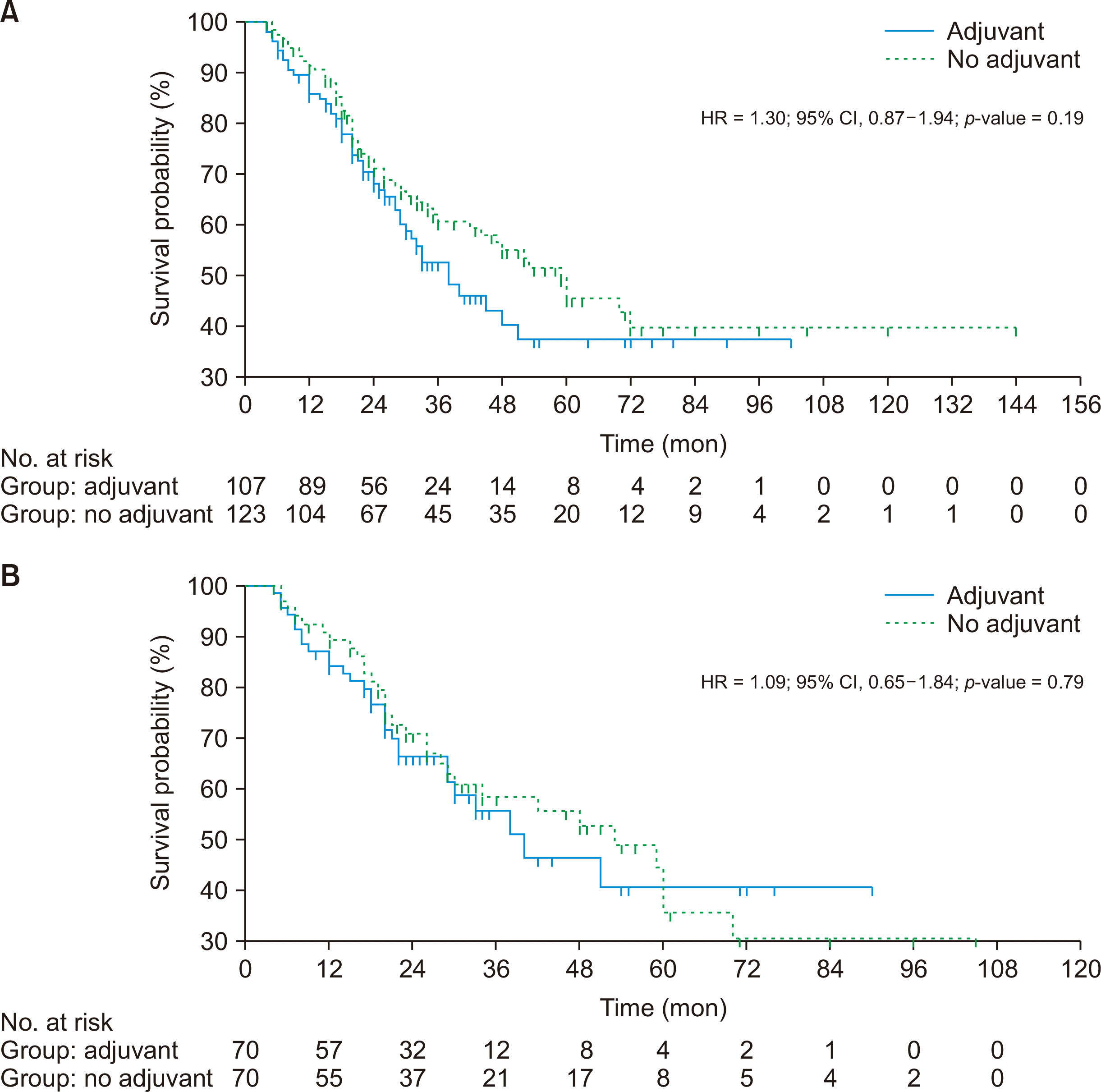
Fig. 4
Forrest plot of various factors for overall survival in a matched population. HR, hazard ratio; CI, confidence interval; PNI, perineural invasion; LNR, lymph node ratio; WD, well differentiation; MD, moderately differentiation; PD, poorly differentiation.
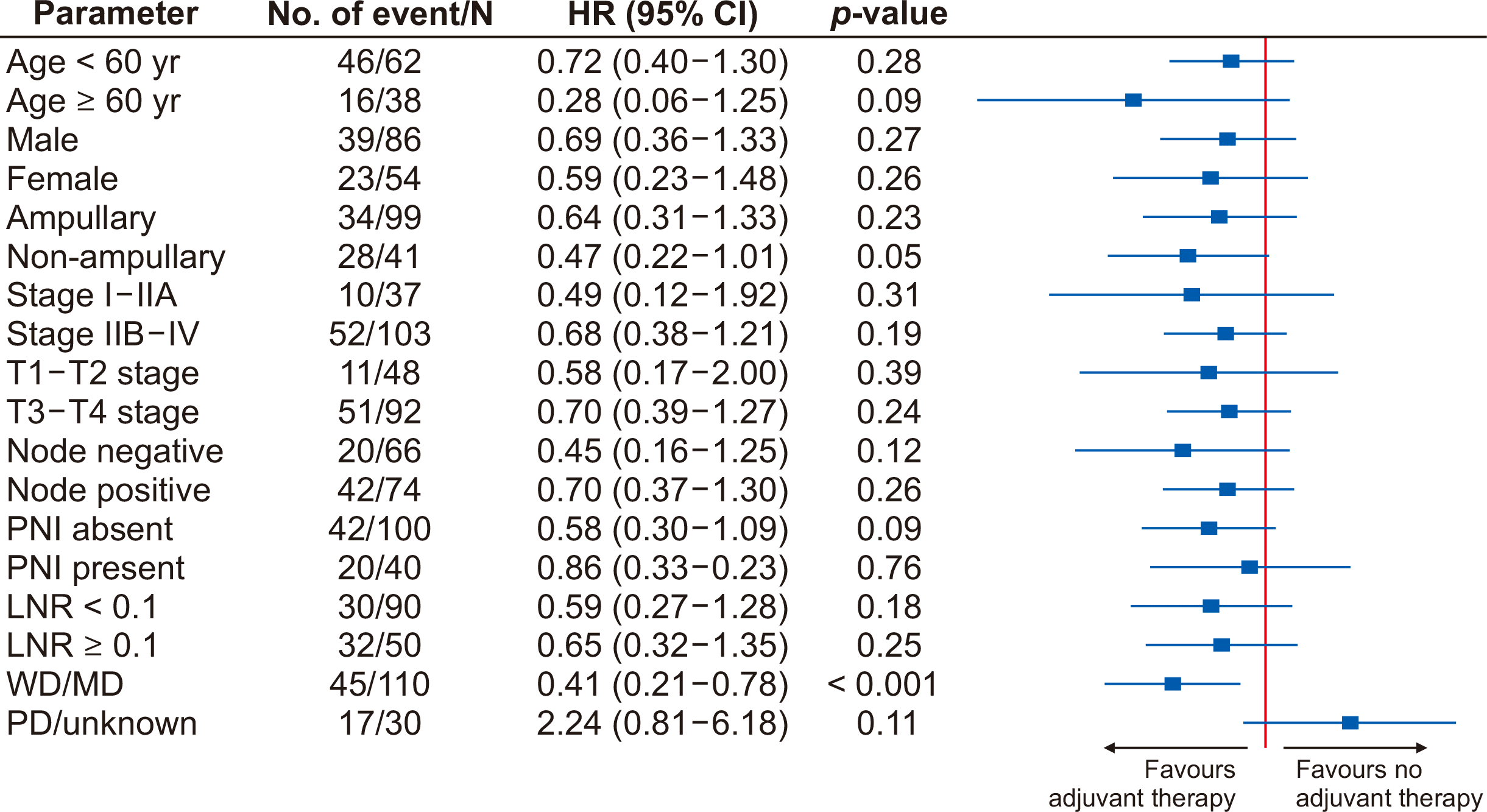
Table 1
Base line and clinical parameters between two groups
| Before matching | After matching | ||||||
|---|---|---|---|---|---|---|---|
| Adjuvant (n = 107) | No adjuvant (n = 123) | p-value | Adjuvant (n = 70) | No adjuvant (n = 70) | p-value | ||
| Age (yr) | 48.1 ± 10.8 | 52.1 ± 11.0 | 0.006* | 47.4 ± 11.0 | 52.5 ± 11.1 | 0.006* | |
| < 60 | 85 (79.4) | 84 (68.3) | 0.056 | 45 (64.3) | 57 (81.4) | 0.023* | |
| ≥ 60 | 22 (20.6) | 39 (31.7) | 25 (35.7) | 13 (18.6) | |||
| Sex | 0.882 | 0.728 | |||||
| Male | 68 (63.6) | 77 (62.6) | 44 (62.9) | 42 (60.0) | |||
| Female | 39 (36.4) | 46 (37.4) | 26 (37.1) | 28 (40.0) | |||
| ECOG | 0.810 | 0.437 | |||||
| 1 | 78 (72.9) | 85 (69.1) | 44 (62.9) | 51 (72.9) | |||
| 2 | 27 (25.2) | 35 (28.5) | 25 (35.7) | 18 (25.7) | |||
| Not mentioned | 2 (1.9) | 3 (2.4) | 1 (1.4) | 1 (1.4) | |||
| Site | 0.270 | 0.578 | |||||
| Biliary/duodenal | 34 (31.8) | 31 (25.2) | 22 (31.4) | 19 (27.1) | |||
| Ampullary | 73 (68.2) | 92 (74.8) | 48 (68.6) | 51 (72.9) | |||
| Differentiation | 0.952 | > 0.999 | |||||
| Well/moderate | 83 (77.6) | 95 (77.2) | 55 (78.6) | 55 (78.6) | |||
| Poor | 24 (22.4) | 28 (22.8) | 15 (21.4) | 15 (21.4) | |||
| T stage | < 0.001* | 0.340 | |||||
| T1 | 0 (0) | 13 (10.6) | 0 (0) | 3 (4.3) | |||
| T2 | 33 (30.8) | 53 (43.1) | 24 (34.3) | 21 (30.0) | |||
| T3 | 68 (63.6) | 54 (43.9) | 42 (60.0) | 43 (61.4) | |||
| T4 | 6 (5.6) | 3 (2.4) | 4 (5.7) | 3 (4.3) | |||
| N0 | 33 (30.8) | 86 (69.9) | < 0.001* | 33 (47.1) | 33 (47.1) | 0.727 | |
| N1 | 53 (49.5) | 29 (23.6) | 26 (37.1) | 29 (41.4) | |||
| N2 | 21 (19.6) | 8 (6.5) | 11 (15.7) | 8 (11.4) | |||
| Overall stage | < 0.001* | 0.565 | |||||
| I-IIA | 17 (15.9) | 64 (52.0) | 17 (24.3) | 20 (28.6) | |||
| IIB-III | 90 (84.1) | 59 (48.0) | 53 (75.7) | 50 (71.4) | |||
| PNI absent | 81 (75.7) | 93 (75.6) | 0.987 | 53 (75.7) | 47 (67.1) | 0.262 | |
| PNI present | 26 (24.3) | 30 (24.4) | 17 (24.3) | 23 (32.9) | |||
| Margin free | 105 (98.1) | 120 (97.6) | 0.768 | 68 (97.1) | 69 (98.6) | 0.559 | |
| Margins involved | 2 (1.9) | 3 (2.4) | 2 (2.9) | 1 (1.4) | |||
| LNR < 0.1 | 65 (60.7) | 96 (78.0) | 0.004* | 47 (67.1) | 43 (61.4) | 0.480 | |
| LNR ≥ 0.1 | 42 (39.3) | 27 (22.0) | 23 (32.9) | 27 (38.6) | |||
| Chemotherapy | |||||||
| Gem + CIS | 85 (79.5) | 59 (84.2) | |||||
| Capox | 4 (3.7) | 3 (4.2) | |||||
| 5 FU based | 4 (3.7) | 2 (2.8) | |||||
| Unknown | 14 (13.1) | 6 (8.5) | |||||
| Chemotherapy completion | |||||||
| 6 cycles | 70 (65.4) | 50 (71.4) | |||||
| 3–5 cycles | 22 (20.6) | 12 (17.1) | |||||
| 0–2 cycles | 15 (14.0) | 8 (11.4) | |||||
Table 2
Recurrence and survival patterns between two treatment groups
| Before matching | After matching | ||||||
|---|---|---|---|---|---|---|---|
| Adjuvant (n = 107) | No adjuvant (n = 123) | p-value | Adjuvant (n = 70) | No adjuvant (n = 70) | p-value | ||
| Recurrence | 49 (45.8) | 52 (42.3) | 0.592 | 29 (41.4) | 31 (44.3) | 0.733 | |
| Controlled | 58 (54.2) | 71 (57.7) | 41 (58.6) | 39 (55.7) | |||
| Recurrence as per T stage group | 0.014* | 0.347 | |||||
| T1-T2 | 6 (12.2) | 17 (32.7) | 4 (13.8) | 2 (6.5) | |||
| T3-T4 | 43 (87.8) | 35 (67.3) | 25 (86.2) | 29 (93.5) | |||
| Distant failure | 23 (21.5) | 37 (30.1) | 0.064 | 14 (20.0) | 24 (34.3) | 0.070 | |
| LR failure | 12 (11.2) | 5 (4.1) | 7 (10.0) | 2 (2.9) | |||
| LR + distant failure | 10 (9.3) | 7 (5.7) | 4 (5.7) | 3 (4.3) | |||
| Exact site not known | 4 (3.7) | 3 (2.4) | 4 (5.7) | 2 (2.9) | |||
| Time of recurrence | 0.492 | 0.595 | |||||
| ≤ 6 mon | 6 (5.6) | 3 (2.4) | 4 (5.7) | 3 (4.3) | |||
| 7 mon to ≤ 2 yr | 26 (24.3) | 29 (23.6) | 18 (25.7) | 15 (21.4) | |||
| > 2 yr to ≤ 5 yr | 17 (15.9) | 18 (14.6) | 7 (10.0) | 12 (17.1) | |||
| > 5 yr | 0 (0) | 2 (1.6) | 0 (0) | 1 (1.4) | |||
| Alive | 67 (62.6) | 63 (51.2) | 0.082 | 48 (68.6) | 30 (42.9) | 0.002* | |
| Dead | 40 (37.4) | 60 (48.8) | 22 (31.4) | 40 (57.1) | |||
| Median RFS in months (95% CI) | 38 (29–51) | 59 (42–72) | 0.195 | 40 (29–51) | 53 (29–70) | 0.797 | |
| Median OS in months (95% CI) | 54 (40–71) | 54 (36–65) | 0.624 | 71 (40–71) | 36 (26–63) | 0.087 | |
Table 3
Univariate and multivariate analysis for overall survival in matched data (n = 140)
| Parameter | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | ||
| Age | |||||
| < 60 yr | Ref | 0.524 | |||
| ≥ 60 yr | 0.83 (0.47–1.45) | ||||
| Sex | |||||
| Female | Ref | 0.900 | |||
| Male | 1.03 (0.61–1.74) | ||||
| Site | |||||
| Ampullary | Ref | < 0.001* | 1.56 (0.90–2.70) | 0.109 | |
| Non-ampullary | 2.83 (1.58–5.04) | ||||
| T stage | |||||
| T1-T2 | Ref | 0.001* | 1.23 (0.60–2.53) | 0.562 | |
| T3-T4 | 2.33 (1.38–3.92) | ||||
| Nodal stage | |||||
| N0 | Ref | < 0.001* | 1.62 (0.74–3.55) | 0.224 | |
| N+ | 2.37 (1.43–3.93) | ||||
| Differentiation | |||||
| WD/MD | Ref | 0.207 | |||
| PD | 1.49 (0.80–2.79) | ||||
| Perineural invasion | |||||
| Absent | Ref | 0.086 | 1.45 (0.82–2.55) | 0.197 | |
| Present | 1.68 (0.92–3.07) | ||||
| Lymph node ratio | |||||
| < 0.1 | Ref | 0.001* | 1.03 (0.49–2.17) | 0.924 | |
| ≥ 0.1 | 2.39 (1.39–4.09) | ||||
| Recurrence | |||||
| None | Ref | < 0.001* | 2.91 (1.56–5.42) | < 0.001* | |
| Yes | 4.64 (2.73–7.87) | ||||
| Adjuvant CT | |||||
| Not given | Ref | 0.087 | 0.69 (0.40–1.19) | 0.188 | |
| Given | 0.64 (0.38–1.06) | ||||
Table 4
Univariate and multivariate analysis for recurrence free survival in matched data (n = 140)
| Parameter | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | ||
| Age | |||||
| < 60 yr | Ref | 0.013* | 0.46 (0.22–0.96) | 0.040* | |
| ≥ 60 yr | 0.48 (0.27–0.84) | ||||
| Sex | |||||
| Female | Ref | 0.996 | |||
| Male | 0.99 (0.58–1.69) | ||||
| Site | |||||
| Ampullary | Ref | 0.003* | 1.43 (0.83–2.45) | 0.190 | |
| Non-ampullary | 2.44 (1.35–4.42) | ||||
| T stage | |||||
| T1-T2 | Ref | < 0.001* | 3.54 (1.49–8.39) | 0.004* | |
| T3-T4 | 3.36 (1.98–5.69) | ||||
| Nodal stage | |||||
| N0 | Ref | < 0.001* | 1.88 (0.87–4.06) | 0.106 | |
| N+ | 2.75 (1.65–4.61) | ||||
| Differentiation | |||||
| WD/MD | Ref | 0.133 | |||
| PD | 1.62 (0.86–3.07) | ||||
| Perineural invasion | |||||
| Absent | Ref | 0.009* | 1.95 (1.11–3.41) | 0.019* | |
| Present | 2.24 (1.22–4.14) | ||||
| Lymph node ratio | |||||
| < 0.1 | Ref | < 0.001* | 1.28 (0.63–2.58) | 0.483 | |
| ≥ 0.1 | 2.65 (1.53–4.61) | ||||
| Adjuvant CT | |||||
| Not given | Ref | 0.719 | |||
| Given | 1.09 (0.65–1.84) | ||||




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download