Abstract
Backgrounds/Aims
We evaluated long-term pancreatic functional outcomes, including pancreatic volumetry after pancreaticoduodenectomy (PD) for peri-ampullary neoplasm.
Methods
We retrospectively reviewed 353 patients with a 12-month follow-up who underwent elective pancreaticoduodenectomies for peri-ampullary neoplasms at a single university hospital between January 2011 and December 2020. Perioperative and postoperative outcomes, long-term pancreatic endocrine functions, and pancreatic volume changes 12 month postoperatively were evaluated.
Results
The mean age was 65.4 years, and the sex ratio was 1.38. The patients with prediagnosed diabetes mellitus (DM) comprised 31.4%. The peri-ampullary neoplasm origins were: the pancreas (49.0%), common bile duct (27.2%), ampulla of Vater (18.4%), and duodenum (5.4%). The 1-week, and 3-, 6-, and 12-month postoperative proportions of patients with DM diagnosed before surgery combined with new-onset postoperative DM were 39.7%, 42.8%, 43.9%, and 49.6%, respectively. The preoperative and postoperative 1-week, and 3-, 6-, and 12-month mean pancreatic volumes were 82.3, 38.7, 28.1, 24.9, and 25.5 mL, respectively. Univariate risk factor analyses for new-onset DM after PD observed no significant difference between the ‘No DM after PD’ and ‘New-onset DM after PD’ groups.
Pancreaticoduodenectomy (PD) is a complex surgical procedure that is performed to treat peri-ampullary neoplasms, including tumors of the pancreas, duodenum, ampulla of Vater, and distal common bile duct [1-5]. While this surgical intervention offers potential curative benefits, it often leads to the loss of a significant portion of the pancreas, which can result in pancreatic insufficiency and endocrine dysfunction [3,6-8].
Understanding the impact of PD on the residual pancreatic volume and endocrine function is essential to optimize patient management and improve postoperative outcomes [6]. Moreover, the endocrine function of the pancreas has a profound effect on the patient’s quality of life [9-11]. In recent years, there has been growing interest in evaluating the long-term consequences of pancreatic resection, specifically in peri-ampullary neoplasms [1,3,12]. However, comprehensive studies focusing on pancreatic volume and endocrine function are limited [3,6,13,14]. Large-scale studies have rarely been conducted, because obtaining the volume of the pancreas is difficult.
Pancreatic volume assessment was conducted using various imaging modalities, such as computed tomography (CT) or magnetic resonance imaging, within a specified time frame postsurgery [3,6,15]. By comparing the pre-and postoperative pancreatic volumes, we could quantify the extent of tissue loss, and evaluate its impact on the endocrine function of the pancreas.
This retrospective study aimed to assess the pancreatic volume and endocrine function in patients who underwent PD for peri-ampullary neoplasms. By characterizing the extent of pancreatic tissue loss and its impact on endocrine function, we aimed to identify the potential predictors of postoperative pancreatic endocrine insufficiency, and develop strategies to optimize patient care.
This retrospective study evaluated 353 consecutive patients with at least a 12-month follow-up period who underwent elective PD for peri-ampullary neoplasms at a single medical center between January 2011 and December 2020. Perioperative and postoperative outcomes, long-term endocrine function of the pancreas, and pancreatic volume changes 12 month postoperatively were evaluated. This study was approved by the Institutional Review Board of the Gachon University Gil Medical Center (No. GDIRB2020-306). This study was performed in accordance with the Declaration of Helsinki, and owing to its retrospective nature, the requirement for written informed consent was waived.
Data were collected on the following parameters for each patient: demographic characteristics, postoperative outcomes, including postoperative pancreatic fistula (POPF), adjuvant chemotherapy, length of hospitalization, HbA1c levels, and serial pancreatic volumes. POPF was defined according to the International Study Group on Pancreatic Fistula (ISGPF) criteria, which define a pancreatic fistula as a measurable volume of drainage fluid with an amylase concentration > 3 times the upper limit of normal after postoperative day 3. Three different grades of POPF were defined according to the clinical impact of POPF on the patient’s postoperative course [16].
Endocrine function data was prospectively collected on the day before surgery, and 1 week and 3, 6, and 12 months after surgery. The endocrine function was evaluated by measuring the fasting blood glucose (FBG) and serum HbA1c levels. Glycemic control was classified as normal, impaired fasting glucose, or diabetes mellitus (DM), according to the American Diabetes Association guidelines [17]. Impaired fasting glucose was defined as FBG levels > 100 mg/dL, while DM was defined as FBG levels > 126 mg/dL [17]. Endocrine function impairment was defined as a deterioration in endocrine function control capacity, as shown by a change from normal before surgery to DM after surgery [17,18].
The patients underwent pancreas-protocol 3-mm thick three-phase contrast-enhanced axial and coronal CTs preoperatively and at 1 week, and 3, 6, and 12 months postoperatively. The pancreatic volume was calculated using the AVIEW program (Version 1.1; Coreline Soft Co., Ltd.) (Fig. 1).
The percentage change in total pancreatic volume at 12 months after surgery was calculated as follows [6]: = [(pancreatic volume at 12 months–preoperative pancreatic volume)/preoperative pancreatic volume] × 100
The percentage change in the remnant pancreatic volume at 12 months after surgery was calculated as follows [6]: = [(pancreatic volume at 12 months–pancreatic volume at 1 week)/pancreatic volume at 1 week] × 100
The primary outcome measured in this study was the percentage change in the remnant pancreatic volume at 12 months after surgery. The secondary outcomes were endocrine function, overall morbidity, and clinically relevant POPF rates.
The operations were performed by three specialized hepato-biliary-pancreatic surgeons. Double-layer duct-to-mucosa pancreaticojejunostomy (PJ) technique was performed in all cases. External or internal PJ stent was inserted in all PJ anastomosis, and the type of stent was decided by the surgeon at the time of operation.
The patient demographics and perioperative and postoperative outcomes were summarized using descriptive statistics. Continuous variables are presented as the mean ± standard deviation (for normally distributed variables), or the median and range (for non-normally distributed variables). Pancreatic volume was calculated using CT at various time points. The percentage changes in the total pancreatic volume and remnant pancreatic volume at 12 months after surgery were calculated using the formulae provided in the study. Univariate analyses were conducted to identify the potential predictors of new-onset DM after PD. Hazard ratios and p-values were calculated to assess the significance of identified risk factors. All analyses were performed using IBM SPSS Statistics (Version 22.0; IBM Corp.).
Table 1 lists the patient demographics. The mean age was 65.4 years, and the sex ratio was 1.38 (males, n = 205, 58.1%). The proportion of prediagnosed DM was 31.4% (n = 111). The origins of peri-ampullary neoplasms were as follow: the pancreas (n = 173, 49.0%), common bile duct (n = 96, 27.2%), ampulla of Vater (n = 65, 18.4%), and duodenum (n = 19, 5.4%). No significant differences were observed between the ‘No DM after PD’ and ‘New-onset DM after PD’ groups in age, sex, body mass index, American Society of Anesthesiologist classification, or origin of neoplasm.
Table 2 lists the perioperative and postoperative outcomes. The mean operative time was 561 minutes. The clinically relevant POPF rate (grades B and C) was 22.4% (n = 79). A total of 318 patients (90.1%) were diagnosed with peri-ampullary malignant neoplasms, and 220 (62.3%) received adjuvant chemotherapy after PD. Chronic pancreatitis was observed in 10 patients (2.8%). The mean postoperative hospital stay was 20.1 days. No significant differences were observed in operation time, length of postoperative hospital stay, PJ stent, POPF, and proportion of malignancy between the ‘No DM after PD’ and ‘New-onset DM after PD’ groups.
The 1-week, and 3-, 6-, and 12-month postoperative HbA1c levels were 6.8%, 6.8%, 7.1%, and 7.6%, respectively. The 1-week, and 3-, 6-, and 12-month postoperative proportions of patients with DM diagnosed before PD combined with the patients with new-onset DM after PD were 39.7%, 42.8%, 43.9%, and 49.6%, respectively (Table 3). The preoperative and postoperative 1 week, 3-, 6-, and 12-month mean pancreatic volumes were 82.3, 38.7, 28.1, 24.9, and 25.5 mL, respectively (Table 4). The percentage change in total pancreatic volume 12 months after PD was –53.4%, while the percentage change in remnant pancreatic volume 12 months after PD was –37.1%.
Table 5 presents the univariate risk factor analyses for new-onset DM after PD. In the univariate analyses, no significant difference was observed between the ‘No DM after PD’ and ‘New-onset DM after PD’ groups in age, body mass index, operation time, sex ratio, adjuvant chemotherapy, origin of neoplasm, POPF grade B or C, PJ stent type, remnant pancreatic volume at 12 months after surgery, or changes in total and remnant pancreatic volumes at 12 months after surgery.
This retrospective study, evaluating pancreatic volume through pancreatic volumetry and assessing endocrine function in patients undergoing PD for peri-ampullary neoplasms, revealed several significant findings. The focus of this study was on examining the long-term pancreatic functional outcomes, encompassing pancreatic volumetry, in patients who had undergone PD for peri-ampullary neoplasms. Studies with a 12-month follow-up, specifically evaluating endocrine function and pancreatic volume in these patients, are scarce, due to the challenges associated with accurately measuring pancreatic volume [3,6,9].
Pancreatic parenchymal atrophy after pancreatectomy is a common phenomenon, and the mechanisms for the atrophy of the pancreatic parenchyma are as follow [3,6,9]. First, surgical procedures can disrupt the blood supply to the remaining pancreatic tissue. The pancreas relies on a robust vascular network for its function, and any compromise in blood flow can contribute to tissue atrophy. Second, when a significant portion of the pancreas is removed, the remaining tissue may undergo functional changes and adaptation, including a decrease in enzyme production and hormonal regulation. This can contribute to overall atrophy. Third, the surgical procedure itself can lead to changes in the structure of the remaining pancreas. Fibrotic tissue formation and alterations in the architecture of the organ may occur in response to the surgery. While atrophy is a common consequence of pancreatectomy, the degree of atrophy and its impact on overall health can vary, depending on the extent of the surgery, and the underlying condition being treated.
During the 1-year period following PD, approximately 20% of patients experienced new-onset DM. The remnant pancreatic volume also steadily decreased by approximately 37% one year after surgery, with the total volume decreasing by approximately 53%, accounting for the resected pancreas volume. Immediately after PD, most patients reduced their food intake, and some temporarily discontinued DM medications. One month after PD, patients generally tended to regain their presurgery levels of food intake. In subgroup analyses conducted in the current study, no significant differences were observed between the early-onset DM (n = 29) and late-onset DM (n = 20) groups in preoperative and postoperative outcomes.
Jung et al. [9] reported a retrospective study including 122 patients who completed 12 months of follow-up using abdominal CTs and a quality-of-life questionnaire to evaluate remnant pancreatic atrophy following PD. This study reported that malignancy and adjuvant chemo-radiotherapy were associated with the severity of the atrophy. At 12 months postoperatively, quality-of-life scores and nutritional indices were mostly not associated with atrophy, but stool elastase levels decreased significantly, and the incidence of new-onset DM was higher in the severe atrophy group [9]. The development of DM in patients after PD was anticipated to be influenced by the cumulative effects of various factors, including pancreatic volume status, alterations in diet, and chemoradiotherapy. In the present study, risk factor analyses were conducted for new-onset DM in patients after PD. However, malignancy, adjuvant chemotherapy, and pancreatic atrophic changes following PD were found to be irrelevant in this study. This is attributed to the inherent difficulty in controlling variables, such as the postsurgery dietary intake of each patient, their level of physical activity, the type and dosage of anticancer drugs administered, family history of diabetes, and variations in skeletal muscle mass that can impact blood sugar control. Consequently, obtaining statistically significant results in the risk factor analysis was deemed unattainable, suggesting inherent limitations in the study design.
Shin et al. [3] reported a prospective randomized controlled study of 213 patients with long-term morphological changes in the pancreas who underwent PD using pancreatic volumetry according to the pancreatic drainage method. The median percentage change in remnant pancreatic volume over 12 months was 45.2% (range, 3.2%−82.8%) in the external stent group, and 46.6% (range, 6.2%−75.1%) in the internal stent group, a difference that was not statistically significant (p = 0.221) [3]. Factors associated with pancreatic exocrine or endocrine function, including stool elastase levels (p = 0.571) and the new-onset DM rate (p = 0.179), were also comparable between the external and internal stent groups [3]. Since there is not much difference in the degree of change in pancreatic volume depending on external or internal PJ stent, it can be inferred that there will be no difference in new-onset DM incidence. External PJ stent was performed in 285 (80.7%) patients, while internal PJ stent was performed in 68 (19.3%) patients in the current study. No significant difference was observed in PJ stent type between the ‘No DM after PD’ and ‘New-onset DM after PD’ groups in the current study.
The results of this study demonstrate that PD has a significant impact on both pancreatic volumes and endocrine functions. The percentage change in the remnant pancreatic volume 12 months after surgery was –37.1%, indicating a substantial reduction in the size of the remaining pancreas. The percentage of patients diagnosed with new-onset DM after surgery increased over time, with 49.6% being diagnosed at the 12-month follow-up. These findings highlight the potential long-term effects of PD on pancreatic volumes and endocrine functions. Few studies have investigated the causal relationship between pancreatic volume loss and incidence of DM [7,13,14,19].
The present study has several limitations. Firstly, the results are susceptible to biases and limitations inherent in a retrospective design. Secondly, pancreas volume is calculated by manually delineating the region of interest, introducing a potential for error, compared to the actual pancreas volume [20]. To mitigate this, two surgeons and two researchers independently confirmed the region of interest. Thirdly, the current study lacks data pertaining to the exocrine function of the pancreas. Nevertheless, it stands out as one of the few studies that encompasses a large number of patients, tracking changes in pancreatic volume and endocrine function over a 12-month period post-PD.
In conclusion, this retrospective study offers valuable insights into the assessment of pancreatic volumes and endocrine functions in patients who underwent PD for peri-ampullary neoplasms. The current study did not identify a causal relationship between pancreatic endocrine dysfunction and atrophic changes in the pancreas following PD. Large-scale prospective studies are necessary to explore the mechanisms, associations, and consequential effects of decreased pancreatic volume and endocrine dysfunction in the pancreas following pancreatectomy.
ACKNOWLEDGEMENTS
The abstract of this paper was published in European Journal of Surgical Oncology under the title ‘Long-term changes in pancreatic volume and endocrine function after pancreaticoduodenectomy for peri-ampullary neoplasms using pancreas-volumetry: A retrospective single center study’ (https://doi.org/10.1016/j.ejso.2022.11.442).
References
1. Lee DH, Jang JY, Kang JS, Kim JR, Han Y, Kim E, et al. 2018; Recent treatment patterns and survival outcomes in pancreatic cancer according to clinical stage based on single-center large-cohort data. Ann Hepatobiliary Pancreat Surg. 22:386–396. DOI: 10.14701/ahbps.2018.22.4.386. PMID: 30588531. PMCID: PMC6295381.
2. Han Y, Lee H, Kang JS, Kim JR, Kim HS, Lee JM, et al. 2018; Progression of pancreatic branch duct intraductal papillary mucinous neoplasm associates with cyst size. Gastroenterology. 154:576–584. DOI: 10.1053/j.gastro.2017.10.013. PMID: 29074452.
3. Shin YC, Jang JY, Chang YR, Jung W, Kwon W, Kim H, et al. 2019; Comparison of long-term clinical outcomes of external and internal pancreatic stents in pancreaticoduodenectomy: randomized controlled study. HPB (Oxford). 21:51–59. DOI: 10.1016/j.hpb.2018.06.1795. PMID: 30093143.
4. Yeo CJ, Cameron JL, Sohn TA, Lillemoe KD, Pitt HA, Talamini MA, et al. Six hundred fifty consecutive pancreaticoduodenectomies in the 1990s: pathology, complications, and outcomes. Ann Surg. 1997; 226:248–257. discussion 257–260. DOI: 10.1097/00000658-199709000-00004. PMID: 9339931. PMCID: PMC1191017.
5. Fernández-del Castillo C, Rattner DW, Warshaw AL. 1995; Standards for pancreatic resection in the 1990s. Arch Surg. 130:295–299. discussion 299–300. DOI: 10.1001/archsurg.1995.01430030065013. PMID: 7887797.
6. Lee DH, Han Y, Byun Y, Kim H, Kwon W, Jang JY. 2020; Central pancreatectomy versus distal pancreatectomy and pancreaticoduodenectomy for benign and low-grade malignant neoplasms: a retrospective and propensity score-matched study with long-term functional outcomes and pancreas volumetry. Ann Surg Oncol. 27:1215–1224. DOI: 10.1245/s10434-019-08095-z. PMID: 31898101.
7. You DD, Choi SH, Choi DW, Heo JS, Ho CY, Kim WS. 2012; Long-term effects of pancreaticoduodenectomy on glucose metabolism. ANZ J Surg. 82:447–451. DOI: 10.1111/j.1445-2197.2012.06080.x. PMID: 22571457.
8. Yoo D, Hwang S, Kim KH, Ahn CS, Moon DB, Ha TY, et al. 2014; Pancreatic atrophy relative to external versus internal drainage of the pancreatic duct after pylorus-preserving pancreaticoduodenectomy. J Gastrointest Surg. 18:1604–1609. DOI: 10.1007/s11605-014-2583-4. PMID: 25002021.
9. Jung W, Kim H, Kwon W, Jang JY. 2022; Atrophy of remnant pancreas after pancreatoduodenectomy: risk factors and effects on quality of life, nutritional status, and pancreatic function. J Hepatobiliary Pancreat Sci. 29:239–249. DOI: 10.1002/jhbp.949. PMID: 33773065.
10. Kitamura T, Anaguchi-Hirao R, Kouhara H. 2008; Combination of type 2 diabetes and malnutrition worsened by anastomotic stenosis and pancreas atrophy following resection of pancreas head. Intern Med. 47:1225–1230. DOI: 10.2169/internalmedicine.47.0233. PMID: 18591845.
11. Kim H, Yoon YS, Han Y, Kwon W, Kim SW, Han HS, et al. 2020; Effects of pancreatic enzyme replacement therapy on body weight and nutritional assessments after pancreatoduodenectomy in a randomized trial. Clin Gastroenterol Hepatol. 18:926–934.e4. DOI: 10.1016/j.cgh.2019.08.061. PMID: 31520730.
12. Philippe MF, Benabadji S, Barbot-Trystram L, Vadrot D, Boitard C, Larger E. 2011; Pancreatic volume and endocrine and exocrine functions in patients with diabetes. Pancreas. 40:359–363. DOI: 10.1097/MPA.0b013e3182072032. PMID: 21283038.
13. Tomimaru Y, Takeda Y, Kobayashi S, Marubashi S, Lee CM, Tanemura M, et al. 2009; Comparison of postoperative morphological changes in remnant pancreas between pancreaticojejunostomy and pancreaticogastrostomy after pancreaticoduodenectomy. Pancreas. 38:203–207. DOI: 10.1097/MPA.0b013e31818e1772. PMID: 19034058.
14. Fang WL, Su CH, Shyr YM, Chen TH, Lee RC, Tai LC, et al. 2007; Functional and morphological changes in pancreatic remnant after pancreaticoduodenectomy. Pancreas. 35:361–365. DOI: 10.1097/MPA.0b013e3180d0a8d5. PMID: 18090244.
15. Lim SH, Kim YJ, Park YH, Kim D, Kim KG, Lee DH. 2022; Automated pancreas segmentation and volumetry using deep neural network on computed tomography. Sci Rep. 12:4075. DOI: 10.1038/s41598-022-07848-3. PMID: 35260710. PMCID: PMC8904764.
16. Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu Hilal M, Adham M, et al. 2017; The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 years after. Surgery. 161:584–591. DOI: 10.1016/j.surg.2016.11.014. PMID: 28040257.
17. American Diabetes Association. 2014; Diagnosis and classification of diabetes mellitus. Diabetes Care. 37 Suppl 1:S81–S90. DOI: 10.2337/dc14-S081. PMID: 24357215.
18. Hirono S, Tani M, Kawai M, Ina S, Nishioka R, Miyazawa M, et al. 2009; A central pancreatectomy for benign or low-grade malignant neoplasms. J Gastrointest Surg. 13:1659–1665. DOI: 10.1007/s11605-009-0934-3. PMID: 19488821.
19. Hashimoto N, Yasuda T, Haji S, Nomura H, Ohyanagi H. 2003; Comparison of the functional and morphological changes in the pancreatic remnant between pylorus-preserving pancreatoduodenectomy and pancreatoduodenectomy. Hepatogastroenterology. 50:2229–2232.
20. Sakata N, Egawa S, Rikiyama T, Yoshimatsu G, Masuda K, Ohtsuka H, et al. 2011; Computed tomography reflected endocrine function of the pancreas. J Gastrointest Surg. 15:525–532. DOI: 10.1007/s11605-010-1406-5. PMID: 21181561.
Fig. 1
(A) AVIEW software (Version 1.1; Coreline Soft Co., Ltd.) for pancreas volumetry. (B) Pancreas volumetry and 3D reconstruction. (C) The pancreas was segmented manually to define the region of interest using the AVIEW software.
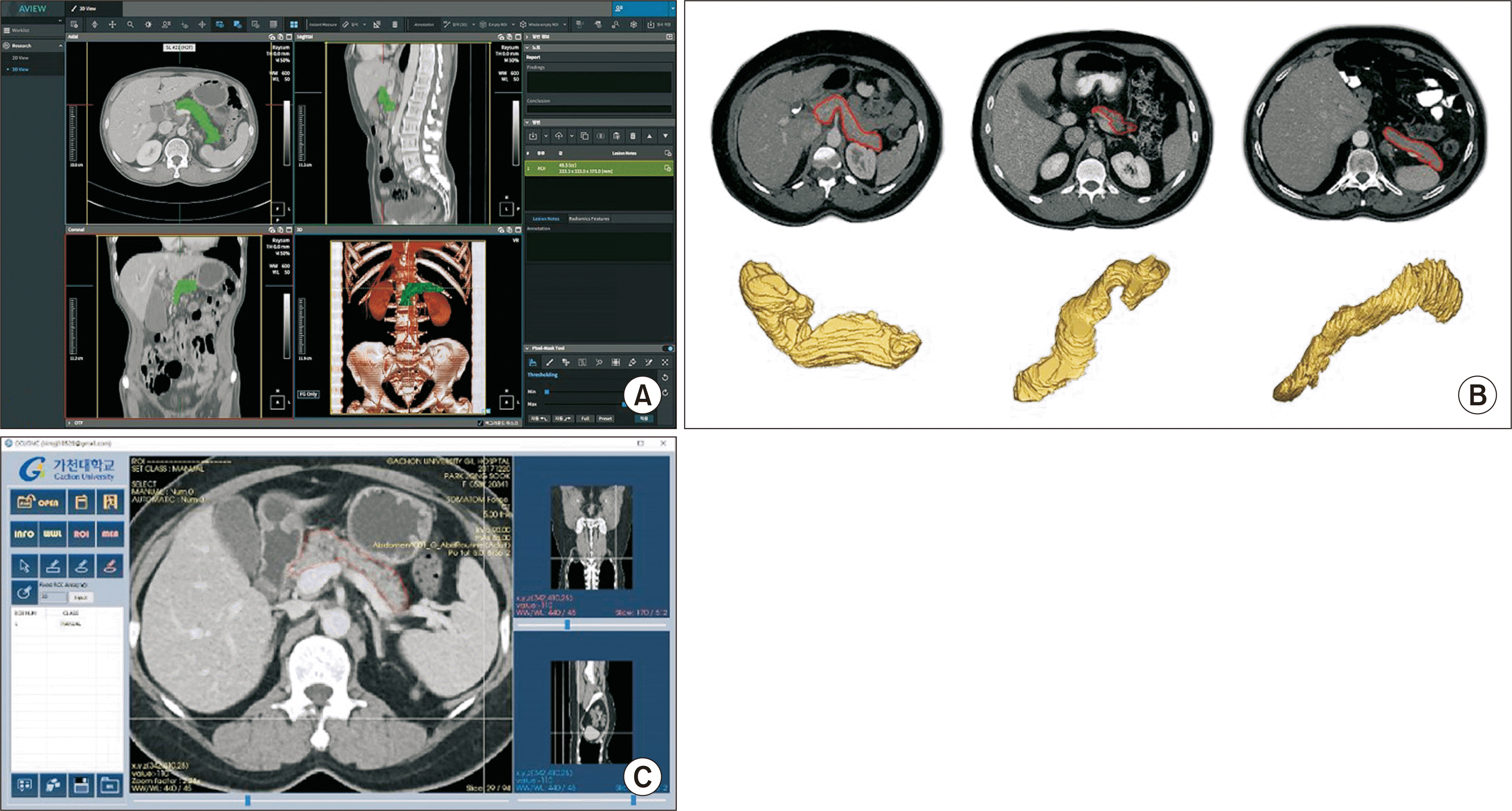
Table 1
Patient demographics
| Total (n = 353) | No DM after PD (n = 178) | New-onset DM after PD (n = 64) | p-valuea) | |
|---|---|---|---|---|
| Age (yr) | 65.4 ± 10.3 | 65.0 ± 10.5 | 63.5 ± 11.0 | 0.327 |
| Sex | 0.461 | |||
| Male | 205 (58.1) | 99 (55.6) | 39 (60.9) | |
| Female | 148 (41.9) | 79 (44.4) | 25 (39.1) | |
| Preoperative body mass index (kg/m2) | 23.2 ± 3.3 | 23.1 ± 3.1 | 23.2 ± 3.1 | 0.893 |
| ASA classification | 0.109 | |||
| 1 | 34 (9.6) | 25 (14.0) | 7 (10.9) | |
| 2 | 296 (83.9) | 146 (82.0) | 50 (78.1) | |
| 3 | 23 (6.5) | 7 (3.9) | 7 (10.9) | |
| Preoperative DM | 111 (31.4) | 0 (0) | 0 (0) | |
| Preoperative hypertension | 164 (46.5) | 107 (60.1) | 38 (59.4) | 0.918 |
| Origin of neoplasm | 0.812 | |||
| Pancreas | 173 (49.0) | 81 (45.5) | 33 (51.6) | |
| Common bile duct | 96 (27.2) | 44 (24.7) | 12 (18.8) | |
| Ampulla of Vater | 65 (18.4) | 38 (21.3) | 12 (18.8) | |
| Duodenum | 19 (5.4) | 11 (6.2) | 5 (3.1) |
Table 2
Perioperative and postoperative patient outcomes
| Total (n = 353) | No DM after PD (n = 178) | New-onset DM after PD (n = 64) | p-valuea) | |
|---|---|---|---|---|
| Operation time (min) | 561.0 ± 101.0 | 560.0 ± 100.9 | 580.3 ± 112.7 | 0.185 |
| Length of postoperative hospital stay (day) | 20.1 ± 18.0 | 20.6 ± 22.4 | 21.6 ± 16.8 | 0.739 |
| Pancreaticojejunostomy stent | 0.563 | |||
| External stent | 285 (80.7) | 145 (81.4) | 50 (78.1) | |
| Internal stent | 68 (19.3) | 33 (18.6) | 14 (21.9) | |
| Postoperative pancreatic fistula | 177 (50.1) | 94 (52.8) | 34 (53.2) | 0.181 |
| Grade A | 98 (27.8) | 56 (31.5) | 14 (21.9) | |
| Grade B | 77 (21.8) | 38 (21.3) | 20 (31.3) | |
| Grade C | 2 (0.6) | 0 (0) | 0 (0) | |
| Grade B or C | 79 (22.4) | 38 (21.3) | 20 (31.3) | |
| Adjuvant chemotherapy | 220 (62.3) | 99 (55.6) | 41 (64.0) | 0.390 |
| Gemcitabine | 150 (42.5) | 60 (33.7) | 30 (46.8) | |
| 5-fluorouracil, leucovorin | 51 (14.4) | 28 (15.7) | 6 (9.3) | |
| 5-fluorouracil, leucovorin, irinotecan, oxaliplatin | 7 (2.0) | 5 (2.8) | 2 (3.1) | |
| Others | 12 (3.4) | 6 (3.3) | 3 (4.7) | |
| Malignancy | 318 (90.1) | 161 (90.4) | 55 (85.9) | 0.317 |
| Combined chronic pancreatitis | 10 (2.8) | 5 (2.8) | 2 (3.1) | 0.345 |
Table 3
Pancreatic endocrine function
Table 4
Pancreatic volumetry
Table 5
Risk factor analysis for new-onset DM in patient after PD




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download