Abstract
Backgrounds/Aims
The efficacy of neoadjuvant transarterial chemoembolization (N−TACE) in resectable hepatocellular carcinoma (HCC) remains open to debate. While N−TACE may reduce tumor size, its impact on long-term outcomes is inconclusive.
Methods
This meta-analysis reviewed studies on N−TACE before surgical resection vs. liver resection (LR) single large hepatocellular carcinoma (SLHCC) up to March 2023 from four online databases.
Results
Five studies with 1,556 patients were analyzed. No significant differences between N−TACE and LR groups were observed in 1-, 3-, or 5-year overall survival (OS) and disease-free survival (DFS). No significant differences were noted in intraoperative blood loss between groups. Subgroup analysis showed favorable 1-, 3-, and 5-year OS with combination chemotherapy N−TACE (combination group), and better 1-year OS in the LR group with single-agent chemotherapy N−TACE (single-agent group). Five-year DFS favored LR in the single-agent group, and N−TACE in the combination group.
Conclusions
Managing SLHCC requires intricate considerations, and the treatment strategies for this challenging subgroup of HCC need to be improved. The influence of N−TACE on long-term survival depends on the specific chemotherapy regimen employed, and its impact on intraoperative blood loss in SLHCC appears limited.
Worldwide, hepatocellular carcinoma (HCC) is the sixth most prevailing form of cancer, and the third foremost cause of cancer-related death. Liver resection (LR) has proven beneficial for disease-free survival (DFS) and overall survival (OS) in early-stage HCC. However, in patients with large HCC (≥ 5 cm), the tumor’s aggressiveness and high recurrence rates following surgery present substantial hurdles, and often result in fatal outcomes [1].
In a minority of patients, primary treatment involves radical resection; nevertheless, significant tumor size and multifocality are recognized as pivotal risk factors that substantially elevate the likelihood of recurrence following HCC resection [2]. Transarterial chemoembolization (TACE) is a locally practical therapeutic approach that can extend OS among individuals with unresectable HCC. Nevertheless, debate on the efficacy of TACE as a neoadjuvant therapy remains ongoing [3]. Several investigations have explored the role of TACE before hepatic resection, yielding varying outcomes [1-10]. These studies are primarily constrained by their limited sample sizes and heterogeneity within the patient sample size. In addition, there is a relative scarcity of randomized controlled trials (RCTs) for this topic. Although data suggests that TACE may induce tumor size reduction, most studies have failed to demonstrate a commensurate enhancement in long-term outcomes. Numerous publications have proposed that preoperative TACE may benefit specific subsets of HCC patients [1,2,4,6]. Therefore, we are conducting this study to determine whether neoadjuvant TACE (N−TACE) followed by LR could improve OS and DFS, rather than a mere resection, in single large hepatocellular carcinomas (SLHCCs).
The present systematic review and meta-analysis are conducted to evaluate the efficacy of N−TACE followed by LR versus upfront LR in SLHCC, by the Preferred reporting items for systematic review and meta-analyses (PRISMA) protocol guidelines. This study protocol was submitted to PROSPERO, the international prospective registry for systematic reviews (ID: CRD42023397769). The 1-, 3-, and 5-year OS and DFS were compared between the two groups as the primary outcome, and intraoperative blood loss between the two groups as the secondary outcome.
The following search phrases and methodology were used to discover the relevant papers in the PubMed, Embase, SCOPUS, and EBSCOhost databases for research published in the English language without any year limit: (“solitary” or “single”) AND (“large” or “huge” or “giant”) AND (“hepatocellular carcinoma” or “HCC” or “Hepatoma” or “liver cancer”) AND (“neoadjuvant” or “preoperative” or “preop”) AND (“transarterial chemoembolization” or “TACE”) AND (“liver resection” or “hepatectomy” or “hepatic resection” or “surgical resection”).
The inclusion criteria were defined by utilizing the Population/Participants, Intervention, Comparison, Outcome, and Study design (PICOS) framework:
• Population: SLHCC patients, defined as patients with a single HCC nodule of ≥ 5 cm in size, regardless of vessel invasion or the involvement of adjacent organs and lymph nodes, in any age, sex, or race who are treated with LR
• Intervention: N−TACE before LR
• Comparison: Upfront LR
• Outcome: 1-, 3-, and 5-year OS and 1-, 3-, and 5-year DFS
• Study design: Prospective and retrospective studies not limited to RCT
Studies that met at least one of the following criteria were excluded: 1) animal studies; 2) case reports; 3) case series; 4) review articles; 5) literature reviews and meta-analyses; 6) studies without a control arm; 7) studies published in languages other than English.
In finding additional research that qualified, the references of the included studies, related reviews, and meta-analyses were manually screened. An assessment for bias was carried out on the included studies to appraise the quality of the study. The Cochrane Risk of Bias 2.0 Assessment Tool was used for studies with RCT designs, while the ROBINS−I tool was used for non-randomized research designs, such as cohorts. Bias risk was evaluated by three investigators (IJ, TJML, and IFA). Discrepancy of opinions during the assessment was resolved through discussions.
The data was statistically analyzed using Cochrane Review Manager 5.4. Studies were divided into the intervention group (N−TACE), and the control group (LR). The 1-, 3-, and 5-year OS and DFS (categorical variable) were analyzed as the primary outcome, along with intraoperative blood loss (numerical variable) as the secondary outcome. The random effect model was used in this meta-analysis. The selected studies were divided into two groups for subgroup analysis based on the chemotherapy regimens used (single agent vs. combination chemotherapy). Heterogeneity (I2) of 25%, 50%, and 75% represent low, middle, and high values, respectively. Forest plots were used to carry out the combined analysis results between studies.
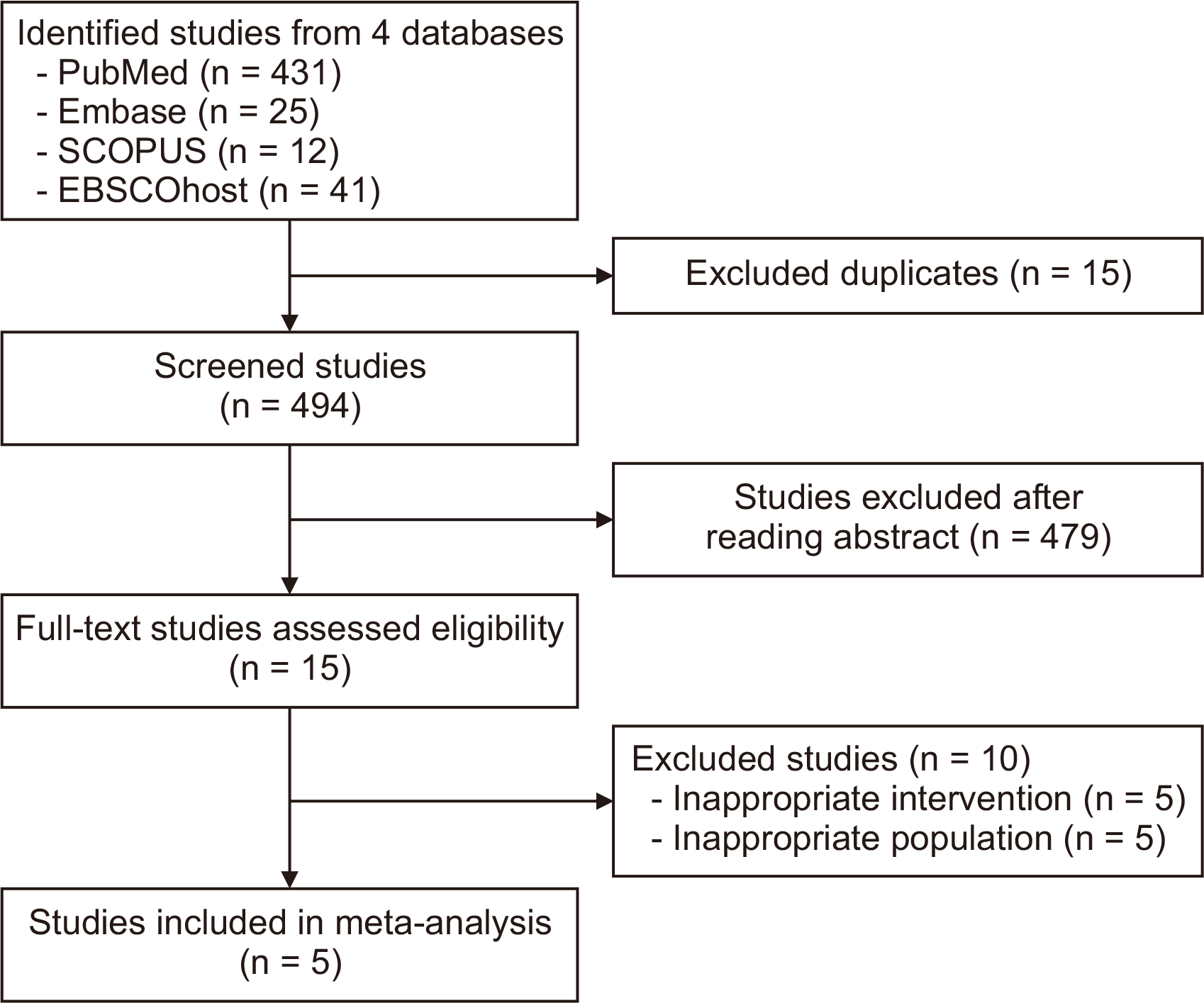
Fig. 1 summarizes the search and screening process of the online medical literature databases. Three cohort retrospectives, one non-randomized comparative prospective study, and a single RCT were included in this meta-analysis. One study used the propensity matching approach for analysis, while the remaining studies were unmatched. Of the 1,556 patients included in the meta-analysis, 474 (30.4%) underwent preoperative TACE (N−TACE) before resection, while 1,082 (69.5%) underwent upfront LR [2-6]. All five studies applied anatomical and non-anatomical LRs to achieve tumor-free margins. Superselective TACE protocols were utilized in all studies; however, the chemotherapy regimens varied [2-6]. Two studies used single chemotherapy agents [2,3] while the others used combination chemotherapy agents [4-6]. Table 1 records the characteristics of the included studies, while Table 2 describes the baseline demographic and oncologic data of the patients enrolled in the included studies. Table 3 states the mortality and complications of the two groups. None of these studies have patients with distant or intrahepatic metastasis. Most demographic data did not significantly differ between N−TACE and LR. Two studies had specific inclusion criteria: the study by Chen et al. [4] (2007) that only included SLHCCs that were centrally located and treated with mesohepatectomy, and the study by Zhang et al. [6] (2015) that only included resectable SLHCCs with portal vein invasion (PVI)/portal vein tumor thrombus (PVTT).
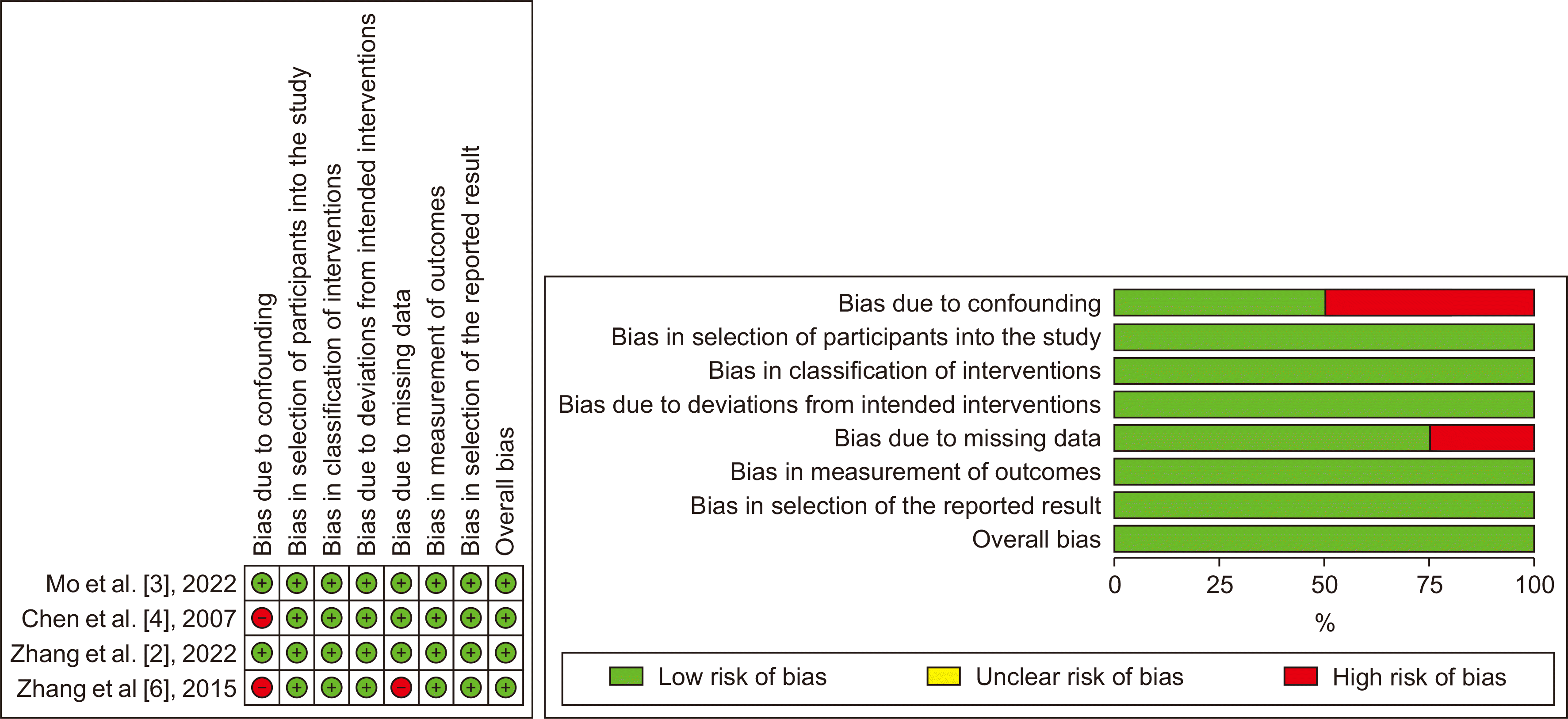
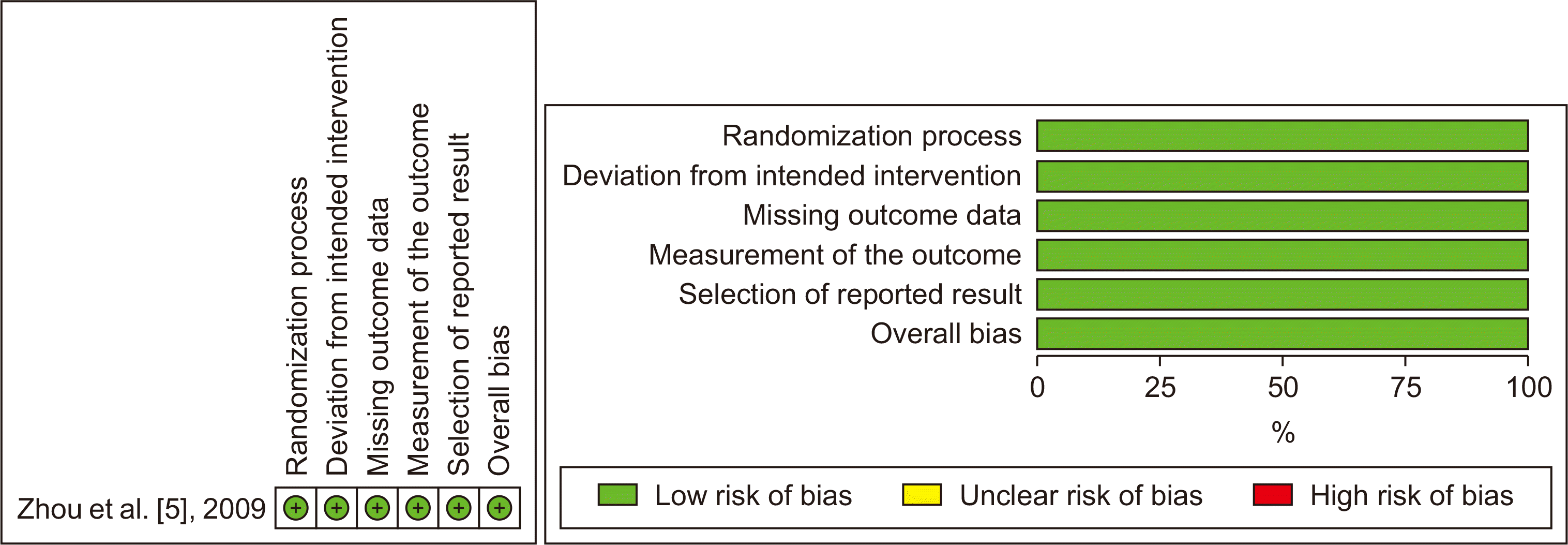
The risk of bias of the four non-randomized studies was assessed using the ROBINS−I tool, while the RCT was assessed using the Risk of Bias (ROB) 2.0, as presented in Fig. 2, 3. Chen et al. [4] and Zhang et al. [6] only included selective SLHCC patients, which could confound this study's outcome. Furthermore, Zhang et al. [6] noted a significant amount of missing data due to the loss of follow-up disease progression, which caused the loss of chance for resection and patient refusal.
The pooled OS was calculated based on the five studies incorporating 1,556 patients (N−TACE = 474 patients; LR = 1,082 patients). No statistically significant differences existed between the N−TACE and LR groups in 1-, 3-, and 5-year OS and DFS. The pooled odds ratios (ORs) for the 1-, 3-, and 5-year OS group were 0.91 (95% confidence interval [CI], 0.54−1.54), 0.80 (95% CI, 0.56−1.15), and 0.88 (95% CI, 0.47−1.65), respectively (Fig. 4). The polled ORs for the 1-, 3-, and 5-year DFS group were 0.66 (95% CI, 0.32−1.34), 0.70 (95% CI, 0.37−1.33), and 0.75 (95% CI, 0.28−1.98), respectively. The heterogeneity test showed high heterogeneity across studies, with I2 of 76%, 55%, and 70% for 1-, 3-, and 5-year OS studies, 89% for 1-year DFS, and 83% for 3- and 5-year DFS studies, respectively (Fig. 5).
The secondary endpoint for this study was to evaluate the intraoperative blood loss between N−TACE and LR. This analysis included two studies with 349 patients (N−TACE = 136, and LR = 213). Statistically significant differences were not observed in intraoperative blood loss between N−TACE and LR (0.64, 95% CI, –0.34–1.62), as seen in the forest plot (Fig. 6). The heterogenicity test showed high heterogeneity (I2 = 94%).
The included studies were allocated into two groups to conduct the subgroup analysis of the primary outcome based on the chemotherapy regimens. Two studies with 917 patients (N−TACE = 253; LR = 664) used single chemotherapy agent studies, while three used combination chemotherapy (639 patients: 221 N−TACE, and 418 LR). There was a statistically significant difference in 1-, 3-, and 5-year OS favoring N−TACE in the combination chemotherapy regimen group, while 1-year OS in the single-agent group tended to favor LR. The heterogenicity test showed low heterogeneity across studies in both groups, as seen in the forest plot (Fig. 7).
There were no statistically significant differences in 1- and 3-year DFS for N−TACE vs. LR in both groups, with low heterogeneity between studies in the single-agent group (I2 = 0%) but high heterogeneity in the combination group (I2 = 87% and 83% for 1- and 3-year, respectively). Meanwhile, 5-year DFS showed statistically favored upfront LR in the single-agent group (OR, 2.82; 95% CI, 1.18−6.72), and favored N−TACE in the combination group (OR, 0.75; 95% CI, 0.28−1.98). The heterogeneity was low across studies in both groups (I2 = 0% and 83% in the single agent and combination groups, respectively) (Fig. 8).
HCC is the leading form of liver cancer globally, accounting for all liver cancer cases [1]. Notably, all studies included in this meta-analysis originate from China, which aligns with prior research indicating a high prevalence of large HCC in Asian populations, particularly in China. A study by Zhang et al. [6] that encompassed data from 75 hospitals and 30,536 HCC patients revealed that 43.1% of patients had tumor sizes exceeding 5 cm, with 22.5% having tumors larger than 10 cm. Consequently, within this meta-analysis, which includes explicitly HCC patients with 5 cm or larger solitary tumors, the entire cohort comprises patients exclusively from China. The presence of satellite nodules was not excluded in our studies, as satellite nodules were defined as small tumors of less than 1 cm in diameter, and a distance of less than 1 cm from the main HCC tumor [1]. Any tumor nodule that appeared more than 1 cm from the primary HCC tumor was excluded. The mean interval time from N−TACE to resection from all the studies we observed was 5.5 weeks for 1 TACE session, and 4.5 weeks for multiple TACE sessions.
Large HCCs are more likely to have microvascular invasion (MVI) or PVI, which are important prognostic risk factors. A significant drawback in the current study is the absence of investigation into PVI. There were only two studies that we examined that indicated PVI. One study by Zhang et al. [6] analyzed the preoperative TACE for resectable HCC with PVI, with 205 patients receiving immediate resection, and 85 patients receiving TACE before resection. That study concludes that N−TACE, particularly for types I and II PVTT, might improve survival for resectable HCC with PVTT. The other study by Zhou et al. [5] assessed preoperative TACE for resectable large HCC with 21 patients showing the presence of PVTT, 11 patients in the N−TACE group, and 10 patients in the control group. In addition, there were other studies within this meta-analysis that reported MVI. In a study by Mo et al. [3], among 556 patients, 359 had MVI, which was more prevalent in Group B, in which the diameter of the tumor was ≥ 10 cm. Chen et al. [4] observed MVI in 40 patients (44.9%) with prior TACE treatment, and 67 patients (42.7%) with only resection. On histopathologic examination, the two groups had no notable distinctions in MVI. Additionally, Zhang et al. [2] noticed that in 220 HCC patients with MVI, a higher positive rate of MVI was found in patients with positive circulating tumor cells.
Some researchers have explored the potential of TACE as a neoadjuvant therapy to enhance the detection of occult intrahepatic metastases, increase resectability by reducing tumor size, and improve the long-term DFS and OS for resectable HCCs [7]. TACE achieves these goals by inducing ischemic necrosis in the tumor through vascular constriction while delivering high-concentration localized chemotherapy for tumor necrosis and size reduction [3,4,7]. Nevertheless, some studies challenge the utility of preoperative TACE in managing resectable HCC. One reason for this skepticism is that TACE primarily affects well-differentiated HCC, while not completely eradicating poorly differentiated cells, which possess a higher malignancy grade, and often spread through the portal venous system [7].
In our survival analysis, this study did not reveal statistically significant differences in 1-, 3-, or 5-year OS and DFS rates between patients who underwent LR with or without N−TACE. These results align with the findings from Jianyong et al. [7], who found that the differences in 1-, 3-, and 5-year OS and DFS rates for resectable HCC patients with or without N−TACE were not statistically significant (p = 0.739 and p = 0.205). Similarly, Nishikawa et al. [11], who researched TACE before surgical resection for HCC patients, discovered that the discrepancies in OS and DFS between the two groups were insignificant, with p = 0.674 and p = 0.062, respectively. A study by Tao et al. [8] involving 152 patients with resectable HCC recurrence that underwent repeated LR with or without preoperative TACE revealed that there were no significant differences in OS (p = 0.407) or DFS (p = 0.791) rates between these two groups. A meta-analysis conducted in 2016 by Si et al. [9] confirmed the impact of N−TACE for resectable HCC; their findings also showed no difference in statistical values between the N−TACE and surgery-only group in OS and DFS. One report showed that preoperative TACE did not affect long-term prognosis, while worsening adhesion. Adhesion in various degrees around the hepatic portal area, thickened gallbladder wall, or shrinkage of the gallbladder was discovered in 20 individuals in the preoperative TACE group. They also identified 15 individuals whose diaphragm was either stuck to the diaphragmatic surface of the liver capsule, or adhered to the tumor. Any of these can cause significant obstacles to surgical procedures [12].
Subgroup analysis within the single-agent chemotherapy group showed no statistically significant differences in 3- and 5-year OS. However, at the 1-year mark, LR exhibited superior outcomes to the N−TACE group. In addition, N−TACE enhanced 1-, 3-, and 5-year OS in the combination agent chemotherapy group. Furthermore, subgroup analysis revealed no statistically significant differences in the 1- and 3-year DFS within the single-agent and combination chemotherapy groups. However, in the 5-year study, LR without N−TACE seemed a more favorable option in the single-agent group, while N−TACE preceding LR was preferred in the combination group.
The efficacy of N−TACE in resectable HCC has been a subject of debate, with studies yielding varying results. Liu et al. [10]’s study utilizing propensity score matching (PSM) analysis demonstrated that LR is associated with significantly better survival than TACE alone for patients with SLHCC (p < 0.001). Consequently, LR should be considered the preferred treatment for individuals with SLHCC. Liang et al. [13] conducted a systematic review and meta-analysis to determine TACE efficacy, rather than the usual LR, in BCLC intermediate stage HCC. They claimed that LR showed significantly better OS in 1, 3, and 5 year than TACE.
Additionally, two RCTs have indicated that preoperative TACE before LR does not improve overall or DFS, aligning with the result presented here [14,15]. Even after matched-pair analysis, two studies proved that the OS is significantly superior in LR than in TACE [16,17]. TACE before surgical resection does not yield superior long-term outcomes, encompassing both OS and DFS. Therefore, considering the cost-effectiveness, N−TACE administration should be avoided in patients with resectable HCC [7].
Nevertheless, it is worth noting that several PSM studies have also reported improved OS in patients with large HCC with preoperative TACE before LR. However, these studies used varying chemotherapy regimens [1,18]. For example, Guo et al. [1] employed a single chemotherapy agent (oxaliplatin, raltitrexed, or epirubicin), and observed improved OS in the PSM survival analysis. Meanwhile, Li et al. [18] used three combination regimens (5–fluorouracil, mitomycin C, cisplatin, carboplatin, doxorubicin, or epirubicin) with similar results. Neither study focused exclusively on SLHCC, but included large HCCs with and without multiple nodules. The latest RCT conducted by Fang et al. [19] in 2023, which also used epirubicin as their single chemotherapy agent, demonstrated improvement in 1-, 2-, and 3-year OS and DFS. Based on the outcomes of this current meta-analysis, it is evident that a combination chemotherapy regimen for N−TACE yields more favorable results than a single-agent N−TACE approach. Supporting our outcomes, Gerunda et al. [20] used epirubicin hydrochloride as their chemotherapy agent. They showed no significant difference on the 1-and 5-year OS between the N−TACE group and the LR group in HCC patients. In their study, Sugo et al. [21] used a single chemotherapy agent, epirubicin or zinostatin stimalamer. They showed that the DFS rates were not statistically significant between patients with and without preoperative TACE.
Drawing upon the comprehensive analysis of the studies detailed herein and the outcomes of this meta-analysis, it is evident that when employed in a combination chemotherapy regimen, N−TACE enhances OS rates in patients with larger HCC. Consequently, implementing a combination N−TACE regimen is advisable for selected patients presenting with larger SLHCC cases, where immediate surgical resection may not be the optimal initial approach. This recommendation underscores the importance of a stratified treatment strategy, highlighting the potential of tailored N−TACE protocols to improve prognostic outcomes in a specific subset of HCC patients.
Among the studies that we have selected and included, only two studies reported the progression rate after N−TACE treatment. In their study, Zhang et al. [6] cvlaimed that from 113 resectable HCC patients with PVI that received N−TACE, 23 showed progressive disease, defined as the growth of PVTT of > 25%. On the other hand, an RCT study run by Zhou et al. [5] stated that 5 of 52 patients in the TACE group could not undergo hepatectomy, as they noticed the disease progression of metastases (n = 4) and liver failure (n = 1). Jianyong et al. [7] also reported tumor progression one year after resection, with 132 cases from the LR group, and 60 cases from the TACE group.
Perioperative mortality data were available from 3 of the 5 studies we observed. Mo et al. [3] reported 4 mortality cases (0.7%) among 556 patients, 2 cases (0.5%) from the LR group, and 2 cases (1.3%) from the N−TACE group. Four instances of in-hospital mortality were identified in a study by Chen et al. [4]: 3 cases (3.4%) in the TACE group with 2 cases from liver failure and 1 case from sepsis, and 1 case (0.6%) in the mesohepatectomy group, which was caused by liver failure. Additionally, Zhang et al. [6] reported treatment mortality, in which 3 cases (1.5%) were found in the LR group, and 1 (0.9%) in the N−TACE group.
Complications, such as perioperative liver failure (PLF) and bile leak, are common in LR with N−TACE cases. Studies from Chen et al. [4], Zhang et al. [6], and Zhou et al. [5] concluded that PLF was seen more in the N−TACE group. However, the other two studies from Mo et al. [3] and Zhang et al. [2] found that PLF was observed more in the LR group. On the other hand, complications of bile leak were found more in LR cases, as almost all the five studies stated, except Chen et al. [4], which observed equal.
The secondary outcome of our study pertains to intraoperative blood loss, which yielded no statistically significant difference. Notably, Chen et al. [4] reported a study in which patients who underwent N−TACE before surgery experienced more significant intraoperative blood loss than those treated with upfront LR, particularly in the context of large centrally located HCC undergoing mesohepatectomy at 790 mL vs. 420 mL [4]. Two studies similar to the previous findings noted that dissection of hepatic parenchyma difficulty is increased in preoperative TACE because of the inflammation, adhesions of perihepatic, or thrombosis of the arterial caused by TACE, resulting in more intraoperative blood loss [22,23]. However, a study conducted in Taiwan contradicted these findings, asserting that there were no statistically significant differences in intraoperative blood loss between patients who received N−TACE before surgery, and those who underwent LR without neoadjuvant therapy (1,468 mL vs. 734 mL, p = 0.62) [14]. Zhou et al. [24] reported that patients who underwent N−TACE, as opposed to LR alone, exhibited a slightly higher mean intraoperative blood loss volume at 652 mL vs. 567 mL. However, the difference was not statistically significant. This outcome may be associated with tumor size, as the N−TACE group in the study had a higher proportion of patients with tumors measuring 10 cm or larger. Again, the difference was not statistically significant [24]. Moreover, several studies stated that when resection is performed approximately one week after TACE, hepatoduodenal ligament thickening and inflammation, collateral neovascularisations, perihepatic adhesions, or chronic cholecystitis can increase intraoperative bleeding [4,25]. In the present study, we detected no statistically significant differences in intraoperative blood loss between the N−TACE and LR groups, although the heterogeneity of the study was high.
Our limitation in this study is that the literature included in the meta-analysis is limited to Chinese populations, which is not globally representative. Conducting similar research with literature from various global centers in the future is highly recommended. In addition, although the studies included were all from China, the heterogeneity of the studies was high. To overcome this heterogeneity, a subgroup analysis was conducted between single-agent and combination-agent chemotherapy, revealing that combination-agent chemotherapy serves a better N−TACE regimen for SLHCC.
The role of N−TACE within the treatment paradigm of SLHCC remains open to debate. The results of this meta-analysis suggest that the efficacy of N−TACE, particularly concerning long-term survival, is based on the specific chemotherapy regimen employed. Notably, using combination-agent chemotherapy within N−TACE protocols yields more favorable outcomes for patients with larger HCCs. Furthermore, this study substantiates the limited effect of N−TACE on reducing intraoperative blood loss. It is evident that the management of SLHCC involves complex considerations, and additional research with a broader international scope is essential to refine treatment strategies, and provide more inclusive insights into the treatment of this challenging group of HCC.
ACKNOWLEDGEMENTS
This research paper owes its success to the invaluable contributions of several individuals. I extend my heartfelt gratitude to Mega Hasanul Huda, PhD, whose expertise in statistical analysis has been instrumental in interpreting our findings. Her insights and suggestions have greatly enhanced the quality of this manuscript. I am also immensely grateful to Ihya Fakhrurizal Amin, MD, for his diligent data collection and analysis. His meticulous work laid the foundation for our study. Furthermore, his role as the second reviewer for the included studies provided an essential layer of scrutiny and academic rigor, ensuring the reliability and validity of our research. Their combined efforts have been pivotal in realizing this project, and I sincerely appreciate their contributions.
References
1. Guo C, Zou X, Hong Z, Sun J, Xiao W, Sun K, et al. 2021; Preoperative transarterial chemoembolization for Barcelona clinic liver cancer stage A/B hepatocellular carcinoma beyond the Milan criteria: a propensity score matching analysis. HPB (Oxford). 23:1427–1438. DOI: 10.1016/j.hpb.2021.02.006. PMID: 33715958.
2. Zhang Q, Xia F, Mo A, He W, Chen J, Zhang W, et al. 2022; Guiding value of circulating tumor cells for preoperative transcatheter arterial embolization in solitary large hepatocellular carcinoma: a single-center retrospective clinical study. Front Oncol. 12:839597. DOI: 10.3389/fonc.2022.839597. PMID: 35664772. PMCID: PMC9159764.
3. Mo A, Zhang Q, Xia F, Huang Z, Peng S, Cao W, et al. 2022; Preoperative transcatheter arterial chemoembolization and prognosis of patients with solitary large hepatocellular carcinomas (≥5 cm): multicenter retrospective study. Cancer Med. 12:7734–7747. DOI: 10.1002/cam4.5529. PMID: 36540041. PMCID: PMC10134378.
4. Chen XP, Hu DY, Zhang ZW, Zhang BX, Chen YF, Zhang WG, et al. 2007; Role of mesohepatectomy with or without transcatheter arterial chemoembolization for large centrally located hepatocellular carcinoma. Dig Surg. 24:208–213. DOI: 10.1159/000102901. PMID: 17522469.
5. Zhou WP, Lai ECH, Li AJ, Fu SY, Zhou JP, Pan ZY, et al. 2009; A prospective, randomized, controlled trial of preoperative transarterial chemoembolization for resectable large hepatocellular carcinoma. Ann Surg. 249:195–202. DOI: 10.1097/SLA.0b013e3181961c16. PMID: 19212170.
6. Zhang YF, Guo RP, Zou RH, Shen JX, Wei W, Li SH, et al. 2015; Efficacy and safety of preoperative chemoembolization for resectable hepatocellular carcinoma with portal vein invasion: a prospective comparative study. Eur Radiol. 26:2078–2088. DOI: 10.1007/s00330-015-4021-8. PMID: 26396105.
7. Jianyong L, Jinjing Z, Wentao W, Lunan Y, Qiao Z, Bo L, et al. 2014; Preoperative transcatheter arterial chemoembolization for resectable hepatocellular carcinoma: a single center analysis. Ann Hepatol. 13:394–402. DOI: 10.1016/S1665-2681(19)30846-4. PMID: 24927610.
8. Tao Q, He W, Li B, Zheng Y, Zou R, Shen J, et al. 2018; Resection versus resection with preoperative transcatheter arterial chemoembolization for resectable hepatocellular carcinoma recurrence. J Cancer. 9:2778–2785. DOI: 10.7150/jca.25033. PMID: 30123345. PMCID: PMC6096377.
9. Si T, Chen Y, Ma D, Gong X, Yang K, Guan R, et al. 2016; Preoperative transarterial chemoembolization for resectable hepatocellular carcinoma in Asia area: a meta-analysis of random controlled trials. Scand J Gastroenterol. 51:1512–1519. DOI: 10.1080/00365521.2016.1216588. PMID: 27598831. PMCID: PMC5152561.
10. Liu P, Su C, Hsu C, Hsia C, Lee YH, Huang YH, et al. 2016; Solitary large hepatocellular carcinoma: staging and treatment strategy. PLoS One. 11:e0155588. DOI: 10.1371/journal.pone.0155588. PMID: 27176037. PMCID: PMC4866714.
11. Nishikawa H, Arimoto A, Wakasa T, Kita R, Kimura T, Osaki Y. 2013; Effect of transcatheter arterial chemoembolization prior to surgical resection for hepatocellular carcinoma. Int J Oncol. 42:151–160. DOI: 10.3892/ijo.2012.1711. PMID: 23174998.
12. Cui H, Gao QQ, Li YY, Wang F. 2003; Influence of preventive effects of transcatheter arterial chemoembolization on primary hepatocellular carcinoma. J Med Forum. 24:1–3.
13. Liang L, Xing H, Zhang H, Zhong J, Li C, Lau WY, et al. 2018; Surgical resection versus transarterial chemoembolization for BCLC intermediate stage hepatocellular carcinoma: a systematic review and meta-analysis. HPB (Oxford). 20:110–119. DOI: 10.1016/j.hpb.2017.10.004. PMID: 29174493.
14. Wu CC, Ho YZ, Ho WL, Wu TC, Liu TJ, P'Eng FK. 1995; Preoperative transcatheter arterial chemoembolization for resectable large hepatocellular carcinoma: a reappraisal. Br J Surg. 82:122–126. DOI: 10.1002/bjs.1800820141. PMID: 7881929.
15. Yamasaki S, Hasegawa H, Kinoshita H, Furukawa M, Imaoka S, Takasaki K, et al. 1996; A prospective randomized trial of the preventive effect of pre-operative transcatheter arterial embolization against recurrence of hepatocellular carcinoma. Jpn J Cancer Res. 87:206–211. DOI: 10.1111/j.1349-7006.1996.tb03160.x. PMID: 8609071. PMCID: PMC5921067.
16. Zhong JH, Ke Y, Gong WF, Xiang B De, Ma L, Ye XP, et al. 2014; Hepatic resection associated with good survival for selected patients with intermediate and advanced-stage hepatocellular carcinoma. Ann Surg. 260:329–340. DOI: 10.1097/SLA.0000000000000236. PMID: 24096763.
17. Min YW, Lee JH, Gwak GY, Paik YH, Lee JH, Rhee PL, et al. 2014; Long-term survival after surgical resection for huge hepatocellular carcinoma: comparison with transarterial chemoembolization after propensity score matching. J Gastroenterol Hepatol. 29:1043–1048. DOI: 10.1111/jgh.12504. PMID: 24863186.
18. Li C, Da M, Lun W, Han L, Jiong W, Yu J, et al. 2019; Preoperative transcatheter arterial chemoembolization for surgical resection of huge hepatocellular carcinoma (≥ 10 cm): a multicenter propensity matching analysis. Hepatol Int. 13:736–747. DOI: 10.1007/s12072-019-09981-0. PMID: 31486964.
19. Fang C, Luo R, Zhang Y, Wang J, Feng K, Liu S, et al. 2023; Hepatectomy versus transcatheter arterial chemoembolization for resectable BCLC stage A/B hepatocellular carcinoma beyond Milan criteria: a randomized clinical trial. Front Oncol. 13:1101162. DOI: 10.3389/fonc.2023.1101162. PMID: 36923427. PMCID: PMC10010190.
20. Gerunda GE, Neri D, Merenda R, Barbazza F, Zangrandi F, Meduri F, et al. 2000; Role of transarterial chemoembolization before liver resection for hepatocarcinoma. Liver Transpl. 6:619–626. DOI: 10.1053/jlts.2000.8312. PMID: 10980062.
21. Sugo H, Futagawa S, Beppu T, Fukasawa M, Kojima K. 2003; Role of preoperative transcatheter arterial chemoembolization for resectable hepatocellular carcinoma: relation between postoperative course and the pattern of tumor recurrence. World J Surg. 27:1295–1299. DOI: 10.1007/s00268-003-6817-y. PMID: 14574482.
22. Paye F, Jagot P, Vilgrain V, Farges O, Borie D, Belghiti J. 1998; Preoperative chemoembolization of hepatocellular carcinoma: a comparative study. Arch Surg. 133:767–772. DOI: 10.1001/archsurg.133.7.767. PMID: 9688007.
23. Luo YQ, Wang Y, Chen H, Wu MC. 2002; Influence of preoperative transcatheter arterial chemoembolization on liver resection in patients with resectable hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 1:523–526.
24. Zhou Q, Tuo F, Li R, Wang X, Wang J, Huang Z, et al. 2020; Transarterial chemoembolization combined with hepatectomy for the treatment of intermediate-stage hepatocellular carcinoma. Front Oncol. 10:578763. DOI: 10.3389/fonc.2020.578763. PMID: 33251141. PMCID: PMC7672209.
25. Hwang TL, Chen MF, Lee TY, Chen TJ, Lin DY, Liaw YF. 1987; Resection of hepatocellular carcinoma after transcatheter arterial embolization. Arch Surg. 122:756–759. DOI: 10.1001/archsurg.1987.01400190022004. PMID: 3036038.
Fig. 4
Forest plot for OS between N−TACE and LR: (A) 1-year OS, (B) 3-year OS, and (C) 5-year OS. OS, overall survival; N−TACE, neoadjuvant transarterial chemoembolization; LR, liver resection; SE, standard error; CI, confidence interval.
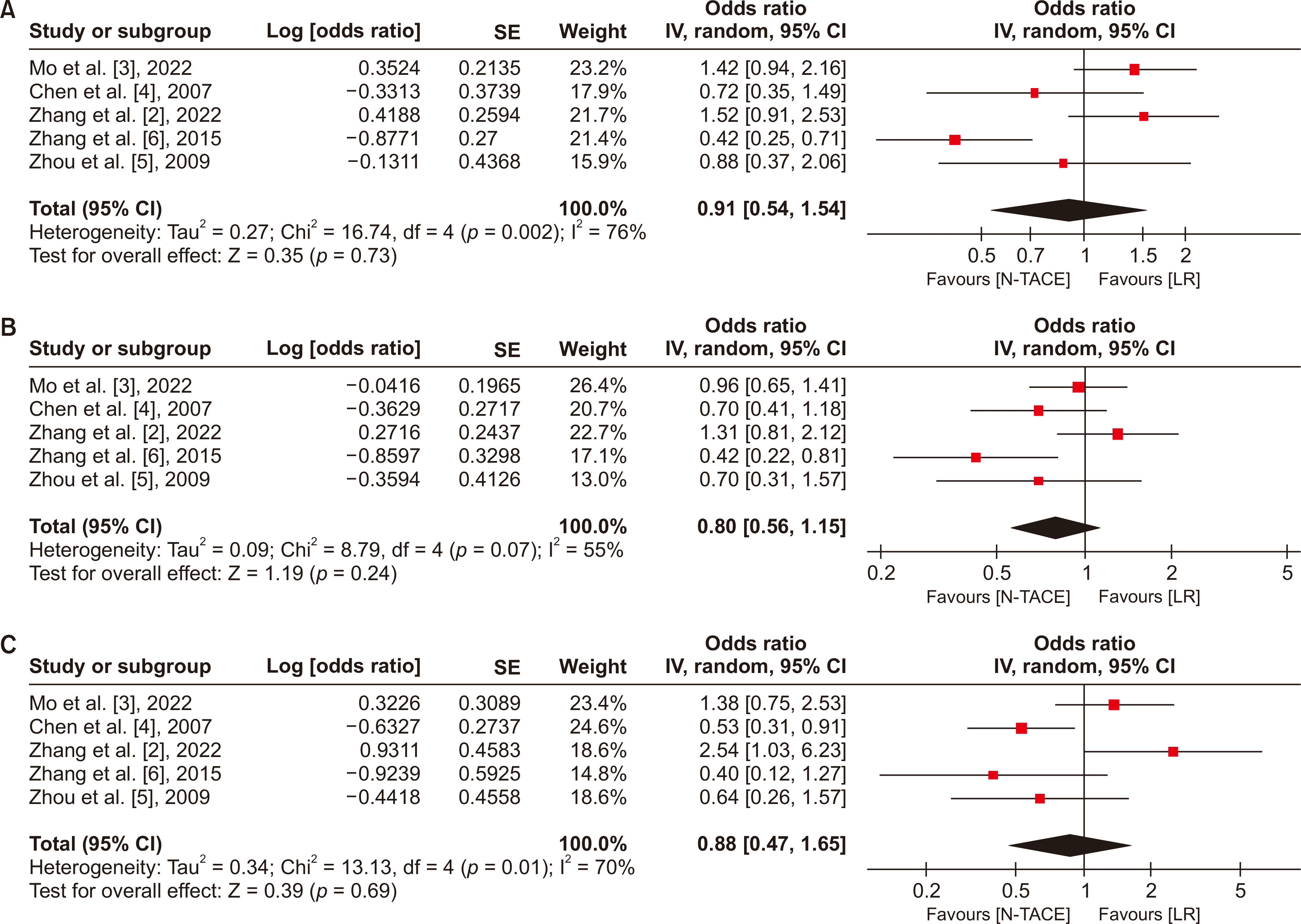
Fig. 5
Forest plot for DFS between N−TACE and LR: (A) 1-year DFS, (B) 3-year DFS, and (C) 5-year DFS. DFS, disease-free survival; N−TACE, neoadjuvant transarterial chemoembolization; LR, liver resection; SE, standard error; CI, confidence interval.
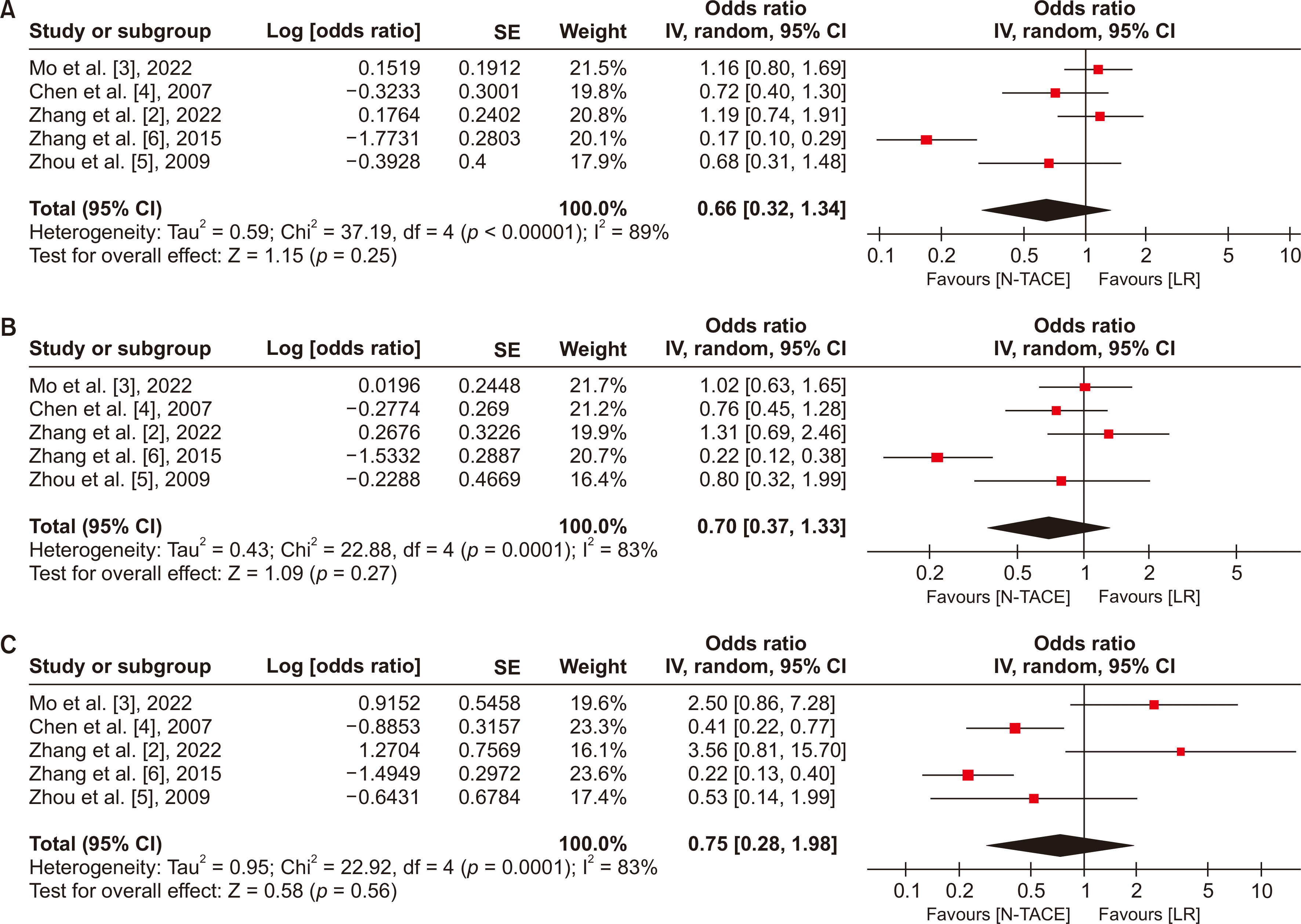
Fig. 6
Forest plot for intraoperative blood loss between N−TACE and LR. N−TACE, neoadjuvant transarterial chemoembolization; LR, liver resection; SD, standard deviation; CI, confidence interval.
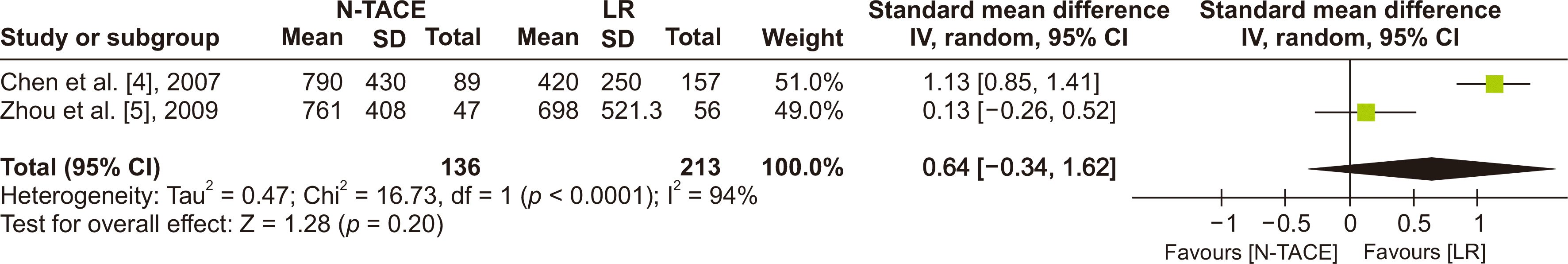
Fig. 7
Forest plot for OS in subgroup analysis based on chemotherapy regimen between N−TACE vs. LR: (A) 1-year OS, (B) 3-year OS, and (C) 5-year OS. OS, overall survival; N−TACE, neoadjuvant transarterial chemoembolization; LR, liver resection; SE, standard error; CI, confidence interval.
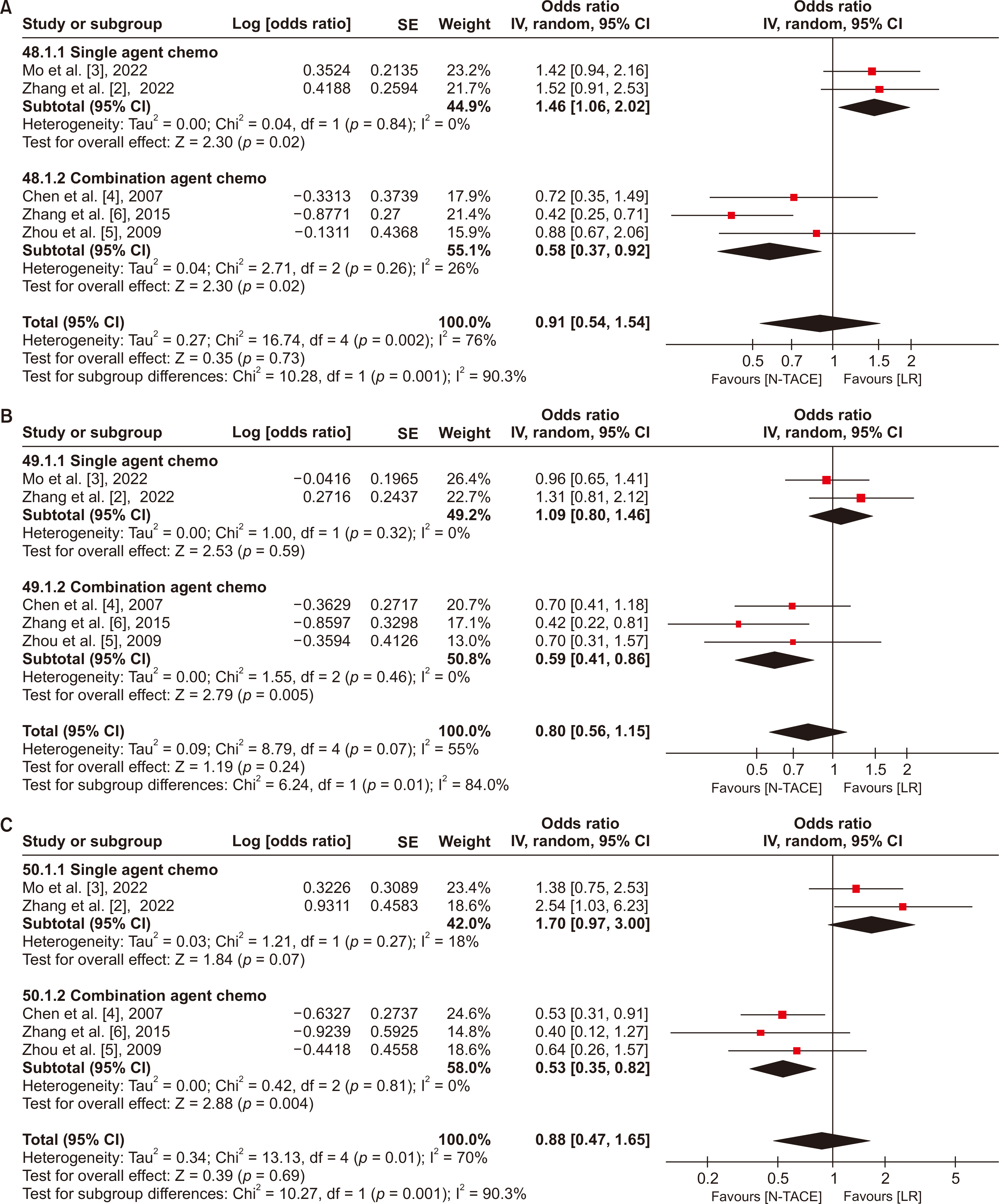
Fig. 8
Forest plot for DFS in subgroup analysis based on chemotherapy regimen between N−TACE vs. LR: (A) 1-year DFS, (B) 3-year DFS, and (C) 5-year DFS. DFS, disease-free survival; N−TACE, neoadjuvant transarterial chemoembolization; LR, liver resection; SE, standard error; CI, confidence interval.
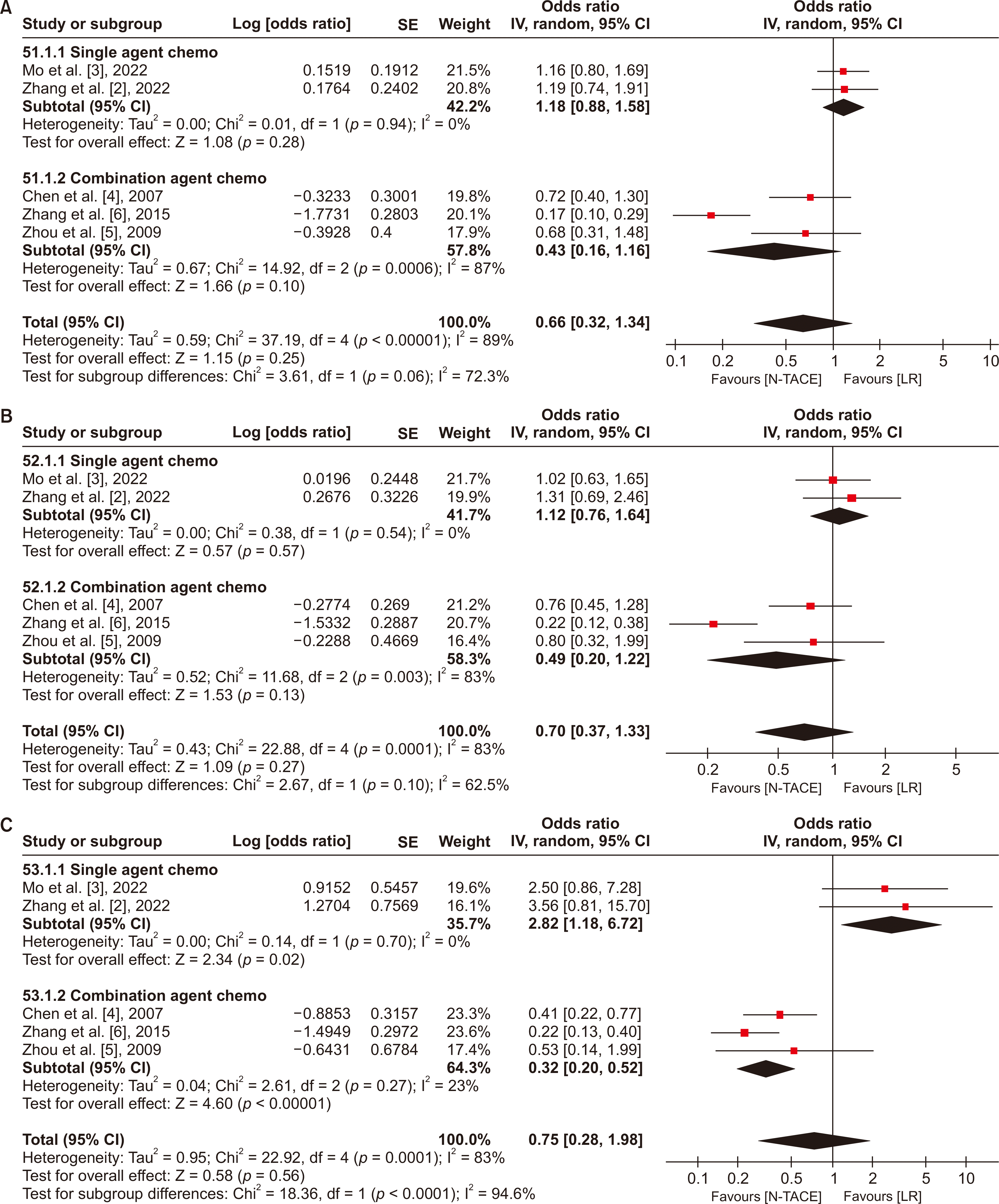
Table 1
Characteristics of the included studies
| Study | Country | Study design | Sample size (N-TACE/LR) | Tumor size (cm) | Mean tumor size (N-TACE/LR) | Regimen TACE | Mean interval N-TACE to surgery (1 session/multiple sessions) | 1-, 3-, 5-yr OS (%) (N-TACE/LR) | 1-, 3-, 5-yr DFS (%) (N-TACE/LR) |
|---|---|---|---|---|---|---|---|---|---|
| Mo et al. [3], 2022 | China | Cohort retrospective | 150/406 | ≥ 5 | 10.0/9.9 | Single: 5-fluorouracil OR oxaliplatin | 5 (4–8) wk/4 (3–6) wk | 70, 39, 10/77, 38, 13 | 48, 18, 6/52, 19, 6 |
| Chen et al. [4], 2007 | China | Cohort retrospective | 89/157 | ≥ 5 | 9.5/9.9 | Combination: 5-fluorouracil, mitomycin C, and cisplatin | NA/26 ± 15 day | 87, 63, 46/82, 54, 32 | 75, 46, 32/70, 38, 17 |
| Zhang et al. [2], 2022 | China | Cohort retrospective | 103/258 | ≥ 5 | NA | Single: 5-fluorouracil OR oxaliplatin | NA/NA | 69, 34, 6/77, 40, 14 | 37, 15, 2/41, 18, 7 |
| Zhang et al. [6], 2015 | China | Non-randomized comparative prospective | 85/205 | ≥ 5 | 9.9/9.1 | Combination: lobaplatin, epirubicin, and mitomycin c | 5 (4–6) wk/5 (4–6) wk | 73, 38, 32/48, 19, 14 | 47, 35, 32/23, 16, 14 |
| Zhou et al. [5], 2009 | China | Randomized controlled trial | 47/56 | ≥ 5 | 9.0/9.5 | Combination: 5-fluorouracil, mitomycin C, cisplatin | 6.5 (5–8) wk/6.5 (5–8) wk | 73, 40, 31/70, 32, 21 | 49, 26, 13/39, 21, 9 |
Table 2
Baseline characteristics of patients in the included studies
| Study | Group | Age (yr) | AFP | Child-Pugh (A/B) | Cirrhosis | HBV/HCV | AR/NAR |
|---|---|---|---|---|---|---|---|
| Mo et al. [3], 2022 | N-TACE | NA | ≤ 400 ug/L = 97 (65); > 400 ug/L = 53 (35) | 128 (85)/22 (15) | 102 (68) | 145 (97)/2 (1) | 57 (38)/93 (62) |
| LR | ≤ 400 ug/L = 258 (64); > 400 ug/L = 148 (36) | 362 (89)/44 (11) | 273 (67) | 388 (96)/6 (2) | 160 (39)/246 (61) | ||
| Chen et al. [4], 2007 | N-TACE | 45.5 ± 6.3 | NA | 78 (88)/11 (12) | 74 (83) | 75 (84)/NA | NA |
| LR | 48.6 ± 5.7 | 142 (90)/15 (10) | 132 (84) | 131 (83)/NA | NA | ||
| Zhang et al. [2], 2022 | N-TACE | NA | ≤ 400 ug/L = 44 (43); > 400 ug/L = 59 (57) | 86 (83)/17 (17) | 73 (71) | 102 (99)/1 (1) | 40 (39)/63 (61) |
| LR | ≤ 400 ug/L = 97 (38); > 400 ug/L = 161 (62) | 227 (88)/31 (12) | 173 (67) | 241(93)/5 (2) | 99 (38)/159 (62) | ||
| Zhang et al. [6], 2015 | N-TACE | 47.9 ± 11.0 | ≤ 400 ug/L = 46 (42); > 400 ug/L = 67 (58) | NA | NA | 105 (91)/3 (3) | 43 (51)/42 (49) |
| LR | 46.8 ± 11.1 | ≤ 400 ug/L = 79 (39); > 400 ug/L = 126 (61) | NA | NA | 194 (95)/2 (1) | 73 (36)/130 (64) | |
| Zhou et al. [5], 2009 | N-TACE | 45.3 ± 9.8 | NA | 44 (85)/8 (15) | 49 (94) | 51 (98)/0 (0) | NA |
| LR | 46.8 ± 9.6 | 54 (96)/2 (4) | 50 (89) | 55 (98)/0 (0) | NA |
Table 3
Mortality and complications of the N−TACE and LR groups
| Study | Peri-operative mortality | Post-hepatectomy liver failure | Bile leak | Intraoperative blood loss | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N-TACE | LR | N-TACE | LR | N-TACE | LR | N-TACE | LR | ||||
| Mo et al. [3], 2022 | 2 (1.3) | 2 (0.5) | 4 (2.7) | 18 (4.4) | 3 (2.0) | 10 (2.5) | NA | NA | |||
| Chen et al. [4], 2007 | 3 (3.4) | 1 (0.6) | 2 | 1 | 1 | 1 | 790 ± 430 | 420 ± 250 | |||
| Zhang et al. [2], 2022 | NA | NA | 2 (1.9) | 15 (5.8) | 1 (1.0) | 9 (3.5) | NA | NA | |||
| Zhang et al. [6], 2015 | 1 (0.9) | 3 (1.5) | 8 | 5 | 2 | 4 | NA | NA | |||
| Zhou et al. [5], 2009 | NA | NA | 5 | 2 | 3 | 4 | 761 ± 408 | 698 ± 521.3 | |||




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download