Abstract
Hepatic compartment syndrome (HCS) is a rare but life-threatening entity that consists of a decreased portal flow due to intraparenchymal hypertension secondary to subcapsular liver hematoma. Lethal liver failure can be observed. We report three cases, and review the literature. A 54-year-old male was admitted for extensive hepatic subcapsular hematoma after blunt abdominal trauma. Initially, he underwent embolization of the hepatic artery’s right branch, after which he presented clinical deterioration, major cytolysis (310 times the upper limit of normal [ULN]), and liver failure with a prothrombin time (PT) at 31.0%. A 56-year-old male underwent liver transplantation for acute alcoholic hepatitis. On postoperative day 2, he presented a hemorrhagic shock associated with deterioration of liver function (cytolysis 21 ULN, PT 39.0%) due to extensive hepatic subcapsular hematoma. A 59-year-old male presented a hepatic subcapsular hematoma five days after a cholecystectomy, revealed by abdominal pain with liver dysfunction (cytolysis 10 ULN, PT 63.0%). All patients ultimately underwent urgent surgery for liver capsule excision, hematoma evacuation, and liver packing, if needed. The international literature was screened for this entity. These three patients’ outcomes were favorable, and all were alive at postoperative day 90. The literature review found 15 reported cases. HCS can occur after any direct or indirect liver trauma. Surgical decompression is the main treatment, and there is probably no place for arterial embolization, which may increase the risk of liver necrosis. A 13.3% mortality rate is reported. HCS is a rare complication of subcapsular liver hematoma that compresses the liver parenchyma, and leads to liver failure. Urgent surgical decompression is needed.
Hepatic compartment syndrome (HCS) is a rare entity corresponding to hypertension in the parenchyma of the liver (> 10−15 mm Hg), resulting in the decrease of portal vein flow and hepatic vein compression [1]. The consequences of HCS can be life-threatening due to acute liver failure, and liver necrosis when associated with low arterial blood flow [2,3]. Only five cases have been described after blunt abdominal trauma [4-6], and none in the early postoperative course of liver transplantation (LT). Diagnosing HCS correctly is essential, since the main differential diagnosis is parenchymal liver injury with perihepatic hematoma, whose management is entirely different. We herein report three cases of HCS. We also review the literature to assess the causes and treatments of this rare entity.
This study was conducted in the Department of HPB Surgery and Liver Transplantation at Beaujon Hospital, Clichy. This tertiary center has an estimated yearly activity of 180 liver resections and 100 LTs. For the literature review, PubMed/Medline research was performed to screen cases of HCS in adult patients, using the following keywords: “hepatic compartment syndrome,” “intrahepatic subcapsular hematoma,” or “compartment syndrome AND liver AND trauma.”
The first patient was a 54-year-old male with no medical history, and appendicitis as the sole surgical history. He was an active smoker, with no alcohol abuse.
He suffered from blunt abdominal trauma of the epigastric area by falling on a trolley. He consulted two days later for increased abdominal pain. He was hemodynamically stable. Blood tests showed a hemoglobin at 8.7 g/dL, elevated transaminase level (5 times the upper limit of normal [ULN]), and normal bilirubin level (26 µmol/L). The initial computed tomography (CT) scan showed an extended subcapsular hematoma without active parenchymal bleeding (Fig. 1A). The patient was admitted to a non-tertiary center for surveillance.
At day 4 post trauma, he became hemodynamically unstable with a mean blood pressure of 65 mm Hg, tachycardia (130/min), and a hemoglobin level of 6.7 g/dL. He was referred to our tertiary center after a transfusion of three red cells units.
A new CT scan showed multiple active subcapsular arterial blushes on the right liver (Fig. 1B). An arteriography (Fig. 1C) showed multiple peripheral blushes. The diagnosis of HCS was not suspected, and the treatment was embolization of the right hepatic artery with gelafoam slurry (Gelitaspon®). Twelve hours after embolization, clinical deterioration was noted with increased abdominal pain. The liver function test showed signs of acute liver dysfunction with low prothrombin time (PT) (30.0%) and Factor V (15.0%), increased bilirubin level (46 µmol/L), and acute cytolysis with elevated aspartate transaminase (AST) (9,658 U/L) and alanine transferase (ALT) (7,215 U/L). An urgent CT scan showed near-complete necrosis of the right liver (Fig. 1D), compression of the right portal vein by the hematoma, and increased volume of the hemoperitoneum. The occurrence of hepatic necrosis due to compression of portal vein by a large subcaspular hematoma after arterial embolization made the diagnosis of HCS. There was no evident major injury in the liver parenchyma.
The patient underwent urgent laparotomy. Surgical exploration showed 4 L of hemoperitoneum, large subcapsular right lobe liver hematoma, and localized ruptured liver capsule. Surgery consisted of hemoperitoneum evacuation, while the liver capsule on the right liver lobe was completely removed to evacuate the subcapsular hematoma, allowing decompression of the hepatic parenchyma. No injury in the liver parenchyma and hemostasis was achieved with bipolar cautery (Fig. 2A, 2B). During surgery, the patient was unstable, requiring vasoactive drugs (3.5 mg/h of norepinephrine) and a transfusion of seven red cell units, three units of fresh plasma, and one unit of platelets. Liver packing and exclusive skin closure was performed. A second look was scheduled at 48 hours, at which time no active bleeding was found, the liver packing was removed, and a cholecystectomy was performed, with an abdominal drainage and complete closure of the abdominal wall.
The postoperative course was favorable, with a progressive decrease in liver transaminases and increased PT. He was extubated at postoperative day (POD) 2. He developed a transient acute renal failure that rapidly normalized, and pneumonia treated by antibiotics. He was discharged home at POD 16. The patient was asymptomatic at two months with normal liver function test and good liver recovery, as shown on the CT scan (Fig. 2C). At 14 months, the patient is asymptomatic with normal liver function, without any delayed complication.
The second patient was a 56-year-old male with a history of asthma, psoriasis, and alcohol abuse. He underwent urgent LT for acute alcoholic hepatitis refractory to medical treatment. He had a Child C13 cirrhosis with a MELD score of 40. He received a brain death donor graft. Cold and warm ischemia times were 510 and 50 minutes, respectively. The surgery required the transfusion of nine red cell units, six units of fresh plasma, and two units of platelets. The initial postoperative course was favorable, with good liver function (PT 55.0%, Factor V 109.0%) and a moderate increase of AST (14 ULN) and ALT (11 ULN) levels on POD 1.
On POD 2, the patient presented hypovolemic shock, requiring the introduction of 1 mg/h of norepinephrine.
Blood tests revealed a decreased hemoglobin level (7 g/dL); liver dysfunction with PT (39.0%), Factor V (69.0%), and bilirubin (255 µmol/L); and acute cytolysis with AST (21 ULN) and ALT (13 ULN). A CT scan showed a large subcapsular hematoma localized in the right liver due to active bleeding in the periphery of the liver, leading to mass effect and compression of the right hepatic vein (Fig. 3A). The inappropriate initial diagnosis was parenchymal liver injury with hematoma. The patient underwent urgent laparotomy with the evacuation of 1.5 L of hemoperitoneum, and liver packing was performed without subcapsular liver hematoma evacuation. He was transfused with four red cell units, two units of fresh plasma, and one unit of platelets. The patient remained unstable postoperatively, requiring 6 mg/h of norepinephrine with a persistent increase in transaminase levels (AST 285 ULN, ALT 66 ULN), low PT (24.0%), and increased bilirubin level (203 µmol/L).
The continuous clinical and biological degradation lead us to consider the diagnosis of HCS related to the presence of liver dysfunction, subcapsular right liver hematoma with parenchymal compression without major parenchymal injury. A repeated urgent laparotomy was performed. Surgical exploration showed hemoperitoneum, large subcapsular right lobe liver hematoma, and localized ruptured liver capsule. The packing was removed, and the hematoma was evacuated after a complete resection of the liver capsule. No major injury was observed in the liver parenchyma (Fig. 3B, 3C, 3D). Hemostasis was completed with bipolar cautery. During surgery, the patient required a transfusion of two red cell units, and three units of fresh plasma.
The postoperative course was rapidly favorable. Norepinephrine was stopped after 24 hours. Liver function improved with Factor V (112.0%) and bilirubin (96 µmol/L) at POD 5, and normalization of the liver enzymes at POD 16. However, the patient required prolonged hospitalization in the intensive care unit due to renal failure and respiratory insufficiency caused by Histoplasmosis infection, with extubation at POD 17. At one month, a CT scan showed good liver recovery (Fig. 3E). The patient died 4 months later from invasive histoplasmosis.
The third patient was a 59-year-old male without medical or surgical history. He underwent a laparoscopic cholecystectomy for symptomatic biliary stones in another center. Postoperative outcome was marked by persistent abdominal pain, which motivated a blood sample on POD 5, which revealed decreased hemoglobin level with hemodynamic stability.
The CT scan showed a large subcapsular hematoma on the right liver (Fig. 4A), and he was referred to our center for urgent care. He underwent transfusion of four red cell units, while blood tests showed low hemoglobin (9 g/dL), cytolysis (AST 10 ULN, ALT 6 ULN), and liver dysfunction (PT 63.0%, bilirubin level 38 µmol/L). The diagnosis of HCS was made combining acute liver dysfunction and postoperative subcapsular right liver hematoma on CT scan without major parenchymal liver injury.
He underwent urgent laparotomy, and surgical exploration showed a large subcapsular hematoma on the anterior and posterior faces of the right lobe without hemoperitoneum with localized rupture of the liver capsule, and without parenchymal liver injury. Surgery consisted of liver capsule excision on the right liver and hematoma evacuation, leading to 2.5 L of blood loss. During the intervention, the patient became unstable with the need for transfusion of 2 red blood cells, and introduction of norepinephrine up to 2.5 mg/h. Liver packing was performed with exclusive cutaneous closure (Fig. 4B). He was reoperated on POD 3 for removal of the packing, completion of the hemostasis, and complete closure of the abdominal wall with abdominal drainage.
Postoperative course was rapidly favorable. Norepinephrine was stopped within 24 hours, and the patient was extubated at POD 1. Hemoglobin remained stable after the initial surgery. Liver function improved promptly (at POD 5 PT 78.0%, Factor V 139.0%, bilirubin 10 µmol/L), and the CT scan showed near evacuation of the hematoma (Fig. 4C). The patient presented a postoperative pulmonary infection with neurological confusion, rapidly favorable with antibiotic treatment. He was discharged home at POD 20. One month after surgery, the patient had normal liver function, was still tired, and not working.
Table 1 summarizes the literature review. Between 1993 and 2022, fifteen cases were described in the literature. The median age (including our cases) was 34 years (interquartile range [IQR]: 27−54), without sex preponderance. The median delay between the triggering event and the diagnosis was 24 hours (IQR: 20−72). Five cases (33.3%) were reported to be related to abdominal trauma [1,4,5], while nine (60.0%) were iatrogenic, secondary to procedures (cholecystectomies or percutaneous procedures) on the liver or gallbladder [3,6-9], or perihepatic surgery on the right kidney, diaphragm, or the right adrenal gland [2,10,11]. The last case was spontaneous [12]. Interestingly, all cases were located on the right liver, and most patients presented hemodynamic instability (69.0%). Nine cases (60.0%) were treated by surgical evacuation and hemostasis, four cases (26.7%) were treated by percutaneous radiologic drainage of the hematoma associated with an arterial embolization when active bleeding was found, and two cases (13.3%) were treated with arterial embolization only. The median hospital stay was 13 days (IQR: 7−28.5), and two patients (13.3%) died.
In our practice of liver surgery and transplantation, subcapsular hematomas may occur during abrupt mobilization of the liver, or tearing of peritoneal attachments. In most cases, these subcapsular hematomas are self-limited, or easily treated by opening the liver capsule, evacuating the hematoma, and performing local hemostasis on the liver parenchyma. In rare cases, without surgical intervention, subcapsular hematomas may enlarge, and cause HCS secondary to parenchymal compression, and a decrease in portal flow [1].
The main differential diagnosis is parenchymal liver injury, a well-known entity with a defined treatment pathway. It is essential to differentiate HCS from liver parenchymal trauma, because the management is completely different. HCS and liver parenchyma injury are two very different entities in terms of clinical and CT-scan presentation. However, HCS is not well known, and can be unnoticed in the emergency care of those patients, leading to inappropriate treatment, as in our first two cases.
First, in parenchymal injury, the delay between the triggering event and the diagnosis was very short, while for subcapsular hematoma with HCS, it was 24 hours (IQR: 20−72) in the literature. Our three cases align with the literature, as symptoms occurred within 48 hours of the triggering event.
The diagnosis of HCS is based mainly on a CT scan that shows a subcapsular hematoma without parenchymal injury, i.e., no sign of liver fracture. Also, biological samples can guide the diagnosis, because HCS is associated with severe acute liver dysfunction and increased liver enzymes due to hepatic veins and portal compression. In contrast, acute liver parenchymal injury will in most cases only be associated with low hemoglobin levels.
While in parenchymal liver injury, the first therapeutic option is conservative (with the help of arterial embolization), and surgery with packing is only recommended for unstable patients, subcapsular hematoma with HCS should be treated by upfront surgical decompression. In most cases, HCS is located on the right liver, and surgery consists of excision of the disrupted liver capsule, evacuation of the hematoma and hemoperitoneum (Fig. 2, 3), and hemostasis of the liver parenchyma by bipolar cautery. Additional packing can be added, especially if the patient is hemodynamically unstable with impaired coagulation.
In our two cases, the diagnosis was not initially evident, explaining the unnecessary embolization in the first case, and the liver packing in the second case. Ultimately, the three patients underwent the same surgical treatment for HCS, i.e., resection of the liver capsule and subcapsular hematoma evacuation.
In HCS, bleeding usually comes from small subcapsular vessels. Literature opinions on the indication of arterial embolization in HCS differ. Indeed, when parenchyma decompression is clearly the main treatment for all authors, the technique use for decompression is either surgical or percutaneous. When percutaneous drainage is chosen by the authors, arterial embolization is said to be necessary when peripherical blushes are visible on CT scan [11,12]. However, for most authors who choose surgical decompression and hemostasis, no arterial embolization was necessary [1,2,5,8-10]. We think there is no indication of arterial embolization, which may paradoxically worsen the patient’s prognosis. Indeed, in those patients with decreased portal and hepatic veins flow, an arterial embolization increases the risk of parenchymal necrosis, and can precipitate liver failure. Furthermore, arterial embolization has several well-known complications that could lead to clinical worsening, such as ischemic cholecystitis and ischemic biliary complications.
Among the reported 15 patients, two (13.3%) ultimately died from multiorgan failure: one after arterial embolization without drainage of a subscapular hematoma [4], and the other after arterial embolization and delayed percutaneous drainage [3]. In the acute phase, evacuating a hematoma containing large clots with radiological percutaneous drainage is difficult. However, this treatment modality can be interesting in the delayed phase after liquefaction of the hematoma, especially in the absence of signs of liver failure.
HCS is a severe complication, and a 13.3% (two patients) mortality rate was reported. Compression of the portal and hepatic veins can lead to liver failure. In the literature, a case of HCS was secondary to a liver graft biopsy after LT, and ultimately needed retransplantation [7]. In two of our three cases, LT or retransplantation was discussed because of the rapid installation of liver failure, and the unknown outcome of this entity after surgery. However, urgent surgical decompression was sufficient to reverse hepatic dysfunction in the acute phase.
HCS is a rare entity that can be lethal, because of a risk of liver failure by compression of the portal and hepatic veins. It can be secondary to any direct or indirect procedure or insult to the right liver. The diagnosis is based on CT scan, and should be differentiated from parenchymal liver injury, usually requiring non-surgical management. The treatment of HCS is urgent surgical decompression with excision of the disrupted liver capsule. There is no indication of arterial embolization, which can increase the risk of liver necrosis and mortality.
References
1. Pearl LB, Trunkey DD. 1999; Compartment syndrome of the liver. J Trauma. 47:796–798. DOI: 10.1097/00005373-199910000-00035. PMID: 10528624.
2. Lacroix G, Boleslawski E, Nzamushe JR. 2022; Hepatic compartment syndrome complicating a right laparoscopic nephrectomy and treated by surgical decompression. J Visc Surg. 159:260–263. DOI: 10.1016/j.jviscsurg.2021.10.007. PMID: 34774450.
3. Marcaire F, Malavieille F, Pichot-Delahaye V, Floccard B, Rimmelé T. 2016; Hepatic compartment syndrome following percutaneous cholecystostomy: a case report. Crit Care Med. 44:e174–177. DOI: 10.1097/CCM.0000000000001403. PMID: 26465220.
4. Markert DJ, Shanmuganathan K, Mirvis SE, Nakajima Y, Hayakawa M. 1997; Budd-Chiari syndrome resulting from intrahepatic IVC compression secondary to blunt hepatic trauma. Clin Radiol. 52:384–387. DOI: 10.1016/S0009-9260(97)80135-3. PMID: 9171794.
5. Petri A, Sándor L, Adám E, Bozó A. 1993; [Acute venous liver circulatory disorder caused by blunt abdominal trauma: is it a compartment syndrome?]. Unfallchirurg. 96:625–627. German.
6. Ye B, Miao YD. 2014; Acute liver failure secondary to hepatic compartment syndrome: case report and literature review. Ulus Travma Acil Cerrahi Derg. 20:136–138. DOI: 10.5505/tjtes.2014.95825. PMID: 24740341.
7. Nissen NN, Geller SA, Klein A, Colquhoun S, Yamini D, Tran TT, et al. 2010; Percutaneous liver biopsy after living donor liver transplantation resulting in fulminant hepatic failure: the first reported case of hepatic compartment syndrome. J Transplant. 2010:273578. DOI: 10.1155/2010/273578. PMID: 20396383. PMCID: PMC2852596.
8. Minaya Bravo AM, González González E, Ortíz Aguilar M, Larrañaga Barrera E. 2010; Two rare cases of intrahepatic subcapsular hematoma after laparoscopic cholecystectomy. Indian J Surg. 72:481–484. DOI: 10.1007/s12262-010-0128-y. PMID: 22131659. PMCID: PMC3077204.
9. Liu QF, Bian LL, Sun MQ, Zhang RH, Wang WB, Li YN, et al. 2019; A rare intrahepatic subcapsular hematoma (ISH) after laparoscopic cholecystectomy: a case report and literature review. BMC Surg. 19:3. DOI: 10.1186/s12893-018-0453-9. PMID: 30616574. PMCID: PMC6323672.
10. Foran CP, Genyk Y, Palmer S, Kim AW. 2020; Hepatic compartment syndrome after a right-sided diaphragm plication. Ann Thorac Surg. 109:e425–427. DOI: 10.1016/j.athoracsur.2019.09.013. PMID: 31606517.
11. Lee HN, Cho SG, Lee WH. 2019; Interventional management of subcapsular hepatic hematoma with hepatic compartment syndrome after laparoscopic adrenalectomy. Cardiovasc Intervent Radiol. 42:625–628. DOI: 10.1007/s00270-018-02158-6. PMID: 30603970.
12. Ando Y, Ishigami M, Ishizu Y, Kuzuya T, Honda T, Hayashi K, et al. 2017; Utility of Doppler ultrasonography for diagnosing and assessing treatment effects in liver compartment syndrome. Clin J Gastroenterol. 10:265–269. DOI: 10.1007/s12328-017-0741-4. PMID: 28447328.
Fig. 1
(A, B) CT showing the subcapsular hematoma without parenchymal injury with active subcapsular bleeding (arrow). (C) The arteriography showing multiple peripheral blushes (arrows). (D) CT few hours after embolization showing complete necrosis of the right lobe and non-visualization of the portal flow. CT, computed tomography.
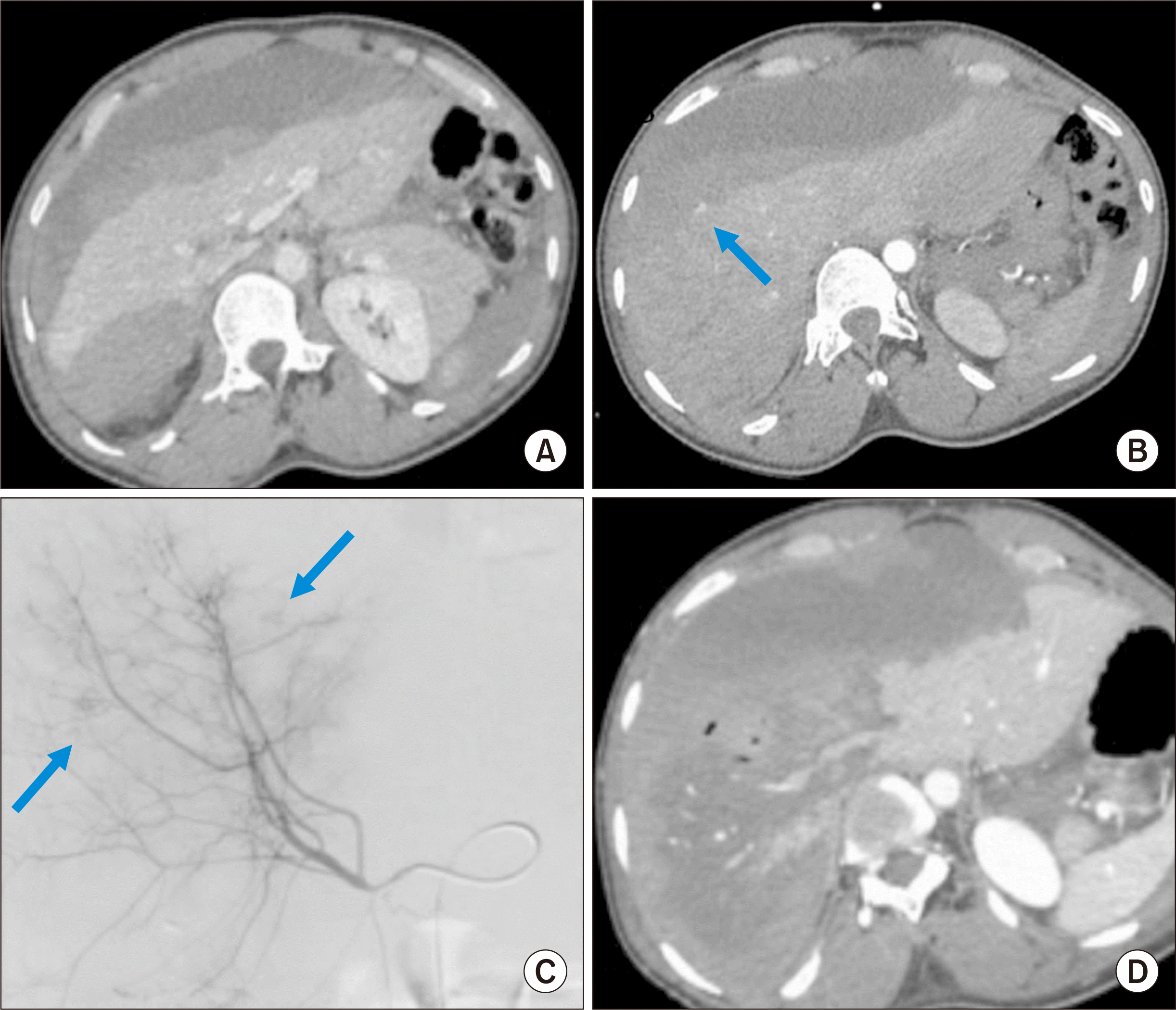
Fig. 2
(A) Intraoperative view showing the subcapsular hematoma (yellow arrow) and the liver capsula (white arrow). (B) The aspect after complete evacuation of the hematoma and resection of the capsula. No injury in the liver parenchyma was noted. (C) Computed tomography at 2 months showed good recovery of the right liver with peristent small foci of necrosis and hypertrophy of the left liver.
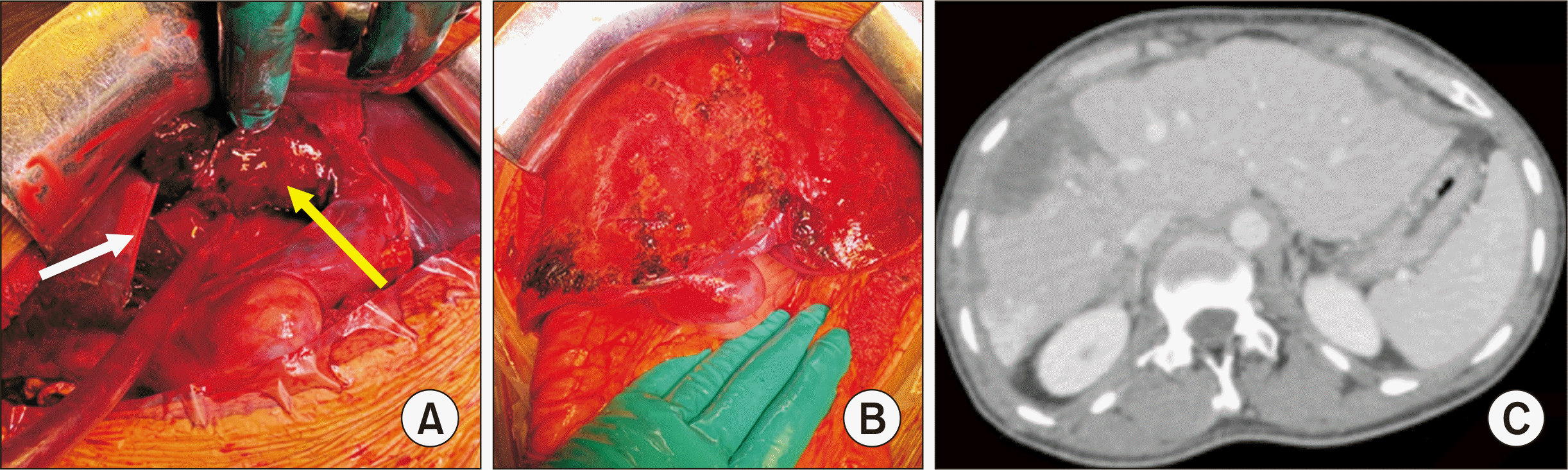
Fig. 3
(A) CT at postoperative day 2 showing subcapsular hematoma with compression of liver parenchyma and of the right hepatic vein. (B, C) Intraoperative view showing the hematoma and ruptured liver capsula. (D) Aspect after liver capsula resection and hemostasis. We can note the absence of parenchymal injury. (E) CT at one month showing good recovery of the right liver parenchyma. CT, computed tomography.
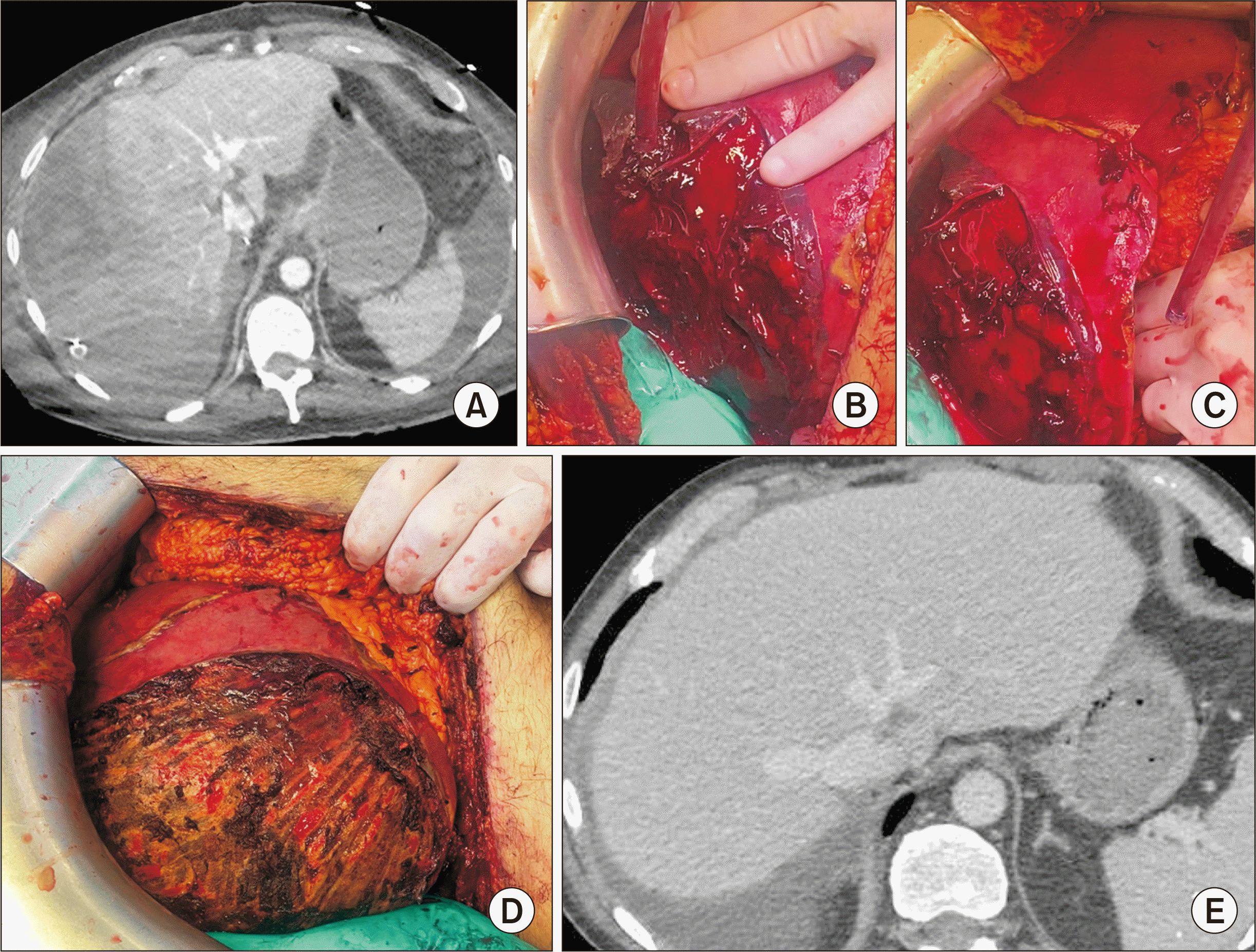
Fig. 4
(A) CT showing a subcapsular hematoma with compression of the liver parenchyma. (B) Intraoperative view showing the aspect after liver capsula resection and liver packing (black arrow). (C) CT at postoperative day 5 showing good recovery of the right liver parenchyma. CT, computed tomography.
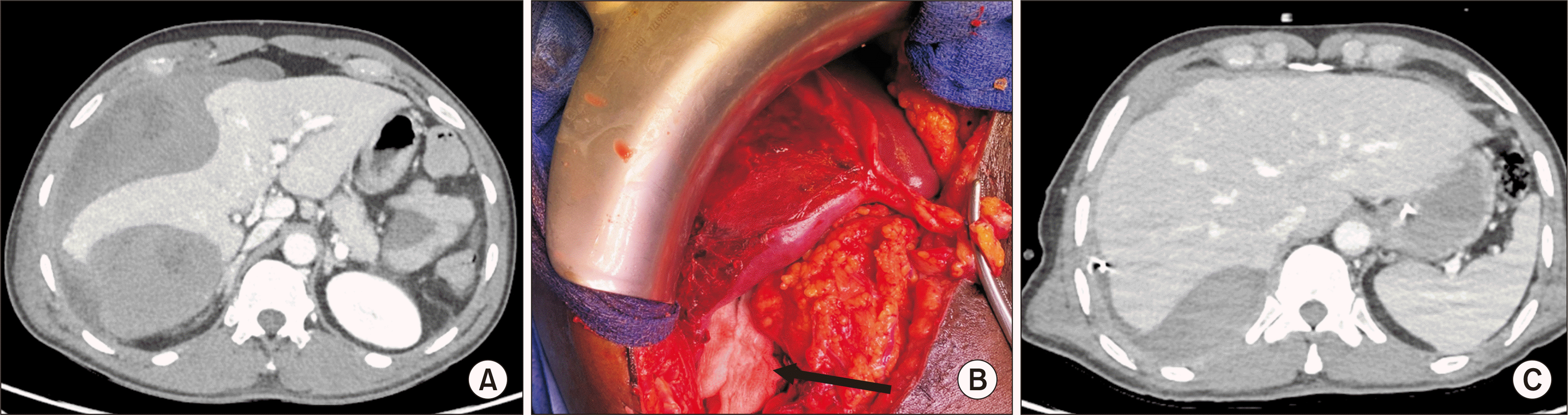
Table 1
Literature description of hepatic compartment syndrome cases
| Author/journal | Age (yr) | Sex | Cause of hepatic compartiment syndrome | Liver side of subcapsular hematoma | Time of occurence after triggering event | Hemodynamic instability | Management | Discharge/death |
|---|---|---|---|---|---|---|---|---|
| Lacroix et al. [2], J Visc Surg, 2022 | 38 | Female | Laparoscopic right nephrectomy | Right | Few h | Yes | Surgery | Discharged (not specified) |
| Foran et al. [10], Ann Thorac Surg, 2020 | 71 | Male | Right diaphragm plication | Right | 20 h | Yes | Surgery | Discharge POD 7 |
| Lee et al. [11], Cardiovasc Intervent Radiol, 2019 | 25 | Male | Laparoscopic right adrenalectomy | Right | 2 day | No | Percutaneous drainage, then embolization of right hepatic artery | Discharge POD 7 |
| Liu et al. [9], BMC Surg, 2019 | 32 | Female | Laparoscopic cholecystectomy | Right | 3 day | Yes | Surgery | Discharge POD 10 |
| Ando et al. [12], Clin J Gastroenterol, 2017 | 32 | Female | Spontaneous | Right | Not applicable | No | Percutaneous drainage and arterial embolization | Discharged (not specified) |
| Marcaire et al. [3], Crit Care Med, 2016 | 64 | Male | Percutaneous cholecystostomy | Right | 15 day | Yes | Embolization of two right sectorial inferior hepatic arteries, then percutaneous drainage | Death 24 h after treatment from multiorgan failure |
| Ye and Miao [6], Ulus Travma Acil Cerrahi Derg, 2014 | 35 | Female | Trauma, with emergency liver surgery | Right | 18 day | No | Percutaneous drainage | Discharge POD 47 |
| Nissen et al. [7], J Transplant, 2010 | 28 | Female | Percutaneous liver biopsy | Right | 20 h | Yes | Transplantation | Discharge POD 13 |
| Minaya Bravo et al. [8], Indian J Surg, 2010 | 69 | Female | Two cases laparoscopic cholecystectomy | Right | 6 day | Yes | Surgery | Discharge POD 37 |
| 29 | Female | Right | 24 h | Yes | Surgery | Discharge POD 30 | ||
| Pearl and Trunkey [1], J Trauma, 1999 | Trauma | Right | 8 h | No | Surgery | Discharge POD 5 | ||
| Markert et al. [4], Clin Radiol, 1997 | 22 | Male | 3 cases after trauma | Right | 24 h | Yes | Surgery | Discharged (not specified) |
| 19 | Male | Right | 31 day | No | Percutaneous drainage | Discharged (not specified) | ||
| 20 | Male | Right | 24 h | Yes | Hepatic arterial embolization | Dead POD 27 | ||
| Petri et al. [5], Unfallchirurg,1993 | 37 | Female | Trauma | Right | < 24 h | Not specified | Surgery | Not specified |
| Case report #1 | 54 | Male | Trauma | Right | 48 h | Yes | Hepatic arterial embolization and surgery | Discharged POD 16 |
| Case report #2 | 56 | Male | Liver transplantation | Right | 48 h | Yes | Surgery | Still hospitalized |
| Case report #3 | 59 | Male | Laparoscopic cholecystectomy | Right | 5 day | No | Surgery | Still hospitalized |
| Median (IQR) | 33.5 (27.5–54.5) | 24 h (20–72) | 13 day (7–28.5) |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download