Abstract
This systematic review and meta-analysis aimed to evaluate the impact of prospective payment systems (PPSs) on cholecystectomy. A comprehensive literature review was conducted, examining studies published until December 2023. The review process focused on identifying research across major databases that reported critical outcomes such as length of stay (LOS), mortality, complications, admissions, readmissions, and costs following PPS for cholecystectomy. The studies were specifically selected for their relevance to the impact of PPS or the transition from fee-for-service (FFS) to PPS. The study analyzed six papers, with three eligible for meta-analysis, to assess the impact of the shift from FFS to PPS in laparoscopic and open cholecystectomy procedures. Our findings indicated no significant changes in LOS and mortality rates following the transition from FFS to PPS. Complication rates varied and were influenced by the diagnosis-related group categorization and surgeon cost profiles under episode-based payment. There was a slight increase in admissions and readmissions, and mixed effects on hospital costs and financial margins, suggesting varied responses to PPS for cholecystectomy procedures. The impact of PPS on cholecystectomy is nuanced and varies across different aspects of healthcare delivery. Our findings indicate a need for adaptable, patient-centered PPS models that balance economic efficiency with high-quality patient care. The study emphasizes the importance of considering specific surgical procedures and patient demographics in healthcare payment reforms.
Global health expenditures have increased since the early 2000s [1], with a significant portion allocated to inpatient care [2]. Managing these escalating costs and fostering quality of care are pivotal concerns, particularly in inpatient provider payment [3]. This area is crucial in shaping how hospitals function as intermediaries between payers and care recipients, impacting financial and healthcare delivery.
The increasing healthcare costs have driven a critical shift in hospital payment methodologies. Traditional retrospective payment models, such as fee-for-service (FFS), are progressively being supplanted by prospective payment systems (PPSs), such as diagnosis-related groups (DRGs) or episode-based payment (EBP) models [4]. In PPS, healthcare providers receive predetermined fixed payments based on clinically relevant classifications [5,6]. These predetermined rates are not influenced by the actual cost or quantity of services provided [7]. This contrasts with the FFS approach, where providers are reimbursed based on the volume and type of services rendered post-treatment. Therefore, PPS shifts financial risk to providers, incentivizing efficiency and cost-effectiveness in care delivery, while FFS potentially leads to increased healthcare costs due to its volume-based incentives [8]. The adoption of PPS aims to mitigate healthcare costs by encouraging providers to optimize resources and focus on value-driven care [9].
The inception of PPS in the United States (USA) in 1983 marked a pivotal response to escalating healthcare costs and economic stagnation, fundamentally restructuring Medicare funding [10]. Since the 1980s, various nations have implemented and refined PPS for inpatient care to optimize resource use and enhance transparency [4,11,12]. Based on similar resource utilization, PPS categorizes patients into groups (e.g., DRGs), assigning predetermined per-case or per-diem rates. This system motivates higher admission rates since marginal revenue decreases beyond the initial treatment day [13]. PPS thus drives a reduction in treatment intensity and per-admission costs. While per-diem systems exhibit milder incentives than per-case systems, the inclination to curtail inputs remains, particularly under a skewed payment schedule [14]. The potential benefits of PPS, such as decreased hospital costs [15,16], shorter length of stay (LOS) [17], and reduced waiting times [18], are offset by paradoxical consequences. These include increased mortality [19], higher readmission rates [20], premature discharge of patients into unprepared community settings [21], and increased administrative healthcare system costs [14,22]. This dichotomy underscores the need for a thorough analysis of PPS’s impact on specific medical procedures, revealing its multifaceted effects on healthcare delivery and patient outcomes.
Cholecystectomy, the surgical excision of the gallbladder, is globally recognized as a standard medical procedure, with laparoscopic cholecystectomy (LC) emerging as the preferred technique [23]. LC is lauded for its minimal invasiveness, characterized by small incisions and the use of a laparoscope, which collectively contribute to shorter patient recovery times and reduced risk of complications [24,25]. Due to its standardized approach and high-frequency occurrence, LC has been seamlessly integrated into the DRG-based PPS worldwide [26]. To date, there is no systematic review of how PPS specifically affects cholecystectomy outcomes. This systematic review seeks to fill this gap by examining the influence of PPS on key aspects of cholecystectomy, including LOS, mortality, hospital admission and readmission rates, and financial impacts. The importance of this study lies in its potential to provide vital insights into the operational effectiveness and financial efficiency of PPS in the context of a frequently performed surgical procedure. Such insights are critical for healthcare policymakers, administrators, and practitioners in shaping and refining healthcare delivery models to align with both economic objectives and patient care priorities.
This study has been registered with PROSPERO (CRD 42024501437) and adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA, 2020) guidelines [27]. A comprehensive literature review was conducted in January 2024 using a combination of keywords: “prospective payment,” “diagnosis-related group*,” “fee-for-service,” “DRG*,” “bundled payment,” “value-based*,” and “cholecystectomy” in PubMed, MEDLINE (Ovid), Embase, and Cochrane Central Register of Controlled Trials. This comprehensive search covered all relevant studies published up to December 2023. The gathered literature was systematically organized using an online reference management tool (Rayyan, Qatar Computing Research Institute,), and duplicate entries were removed. Two reviewers (YZ and IEHT) conducted the literature selection independently, with any discrepancies resolved through consultation with a third author (YXK).
This study aimed to evaluate the impact of PPS on cholecystectomy, guided by a PICOS framework (Population, Intervention, Comparison, Outcome, and Study) [28]. Inclusion criteria were formulated to encompass studies on payment systems relevant to cholecystectomy, aligned with the components of the PICOS framework as follows: 1) Population: Inpatients of any gender and age undergoing cholecystectomy; 2) Intervention: Prospective payment reforms implemented at the hospital level or as part of national/state health initiatives, including both pilot and expanded programs; 3) Comparison: Traditional payment methods used before the implementation of PPS; 4) Outcome: All types of outcomes, whether at the hospital level or individual patient level; 5) Study: All types of observational and experimental study designs.
Additional inclusion criteria were applied: 6) accessibility to full-text articles; 7) articles written in English; 8) exclusion of qualitative studies and narrative reports. Dissertations, editorials, and consensus statements were also excluded.
The main objective of this study was to evaluate the impact of PPS on cholecystectomy outcomes, focusing specifically on LOS and mortality rates (defined as death within 30 or 90 days after cholecystectomy or in-hospital mortality). Additionally, the study examined non-pooled secondary outcomes such as complications, admissions, readmissions, hospital costs, and financial margins.
Data extraction was conducted by the designated screening authors (YZ and IEHT). The information collected from each article included the first author's name, publication year, study country, intervention, data source, population, payment methods, analytical methods, and outcomes. For continuous variables reported as medians with range or interquartile range, means and standard deviations were estimated using specific mathematical algorithms developed by Luo et al. [29] and Wan et al. [30]. Costs were initially recorded in their original currencies and subsequently converted to US dollars ($), factoring in inflation up to December 2023, based on the consumer price index from the Internal Revenue Service of the USA (https://www.irs.gov/individuals/international-taxpayers/yearly-average-currency-exchange-rates).
The risk of bias (RoB) in included studies was evaluated using the ROBINS-I tool (Risk Of Bias In Non-randomized Studies–of Interventions) [31]. Forest plots were scrutinized, and I2 statistics were calculated to assess heterogeneity [32]. Sensitivity analyses were performed on the outcomes demonstrating significant heterogeneity (I2 > 70%). The assessment of publication bias was conducted using funnel plots [33].
In studies presenting data amenable to aggregation, a meta-analysis was conducted to compare the effects of PPS versus traditional payment methods on LOS and mortality. The pooled odds ratio and its 95% confidence interval (CI) were calculated for dichotomous outcomes, while the weighted mean difference with its 95% CI was determined for continuous outcomes. Data from various studies were integrated using a random-effects model, allowing for the compilation of effect estimates. All statistical analyses were conducted using R statistical software (version 4.3.1; R Foundation for Statistical Computing). Other findings were systematically described for studies with data unsuitable for pooling.
The methodology for this systematic review follows the PRISMA guidelines, as illustrated in the PRISMA flowchart (Fig. 1). The initial search yielded a total of 106 records. After a thorough screening, six studies [34-39] specifically examining the impact of PPS on cholecystectomy were identified. These studies originated from various countries between 2002 and 2023: one from Israel, two from Taiwan, two from the USA, and one from Ireland. Detailed information about these studies is shown in Table 1. Three studies [34,35,39] conducted a comparative analysis between FFS and PPS for LOS, and mortality were subsequently included in the meta-analysis. The remaining three studies provided distinct insights: one study [38] evaluated and compared two different DRG-based payment schemes for LC, while the other two studies [36,37] focused on the outcomes following EBP implementation for cholecystectomy. These latter studies were described separately due to their specific focuses. The baseline characteristics of the studies included are shown in Supplementary Table 1.
The assessment of RoB for the included studies is depicted in Supplementary Fig. 1. While certain studies exhibited potential biases, the overall risk assessment across the reviewed literature was deemed within acceptable limits. Significant heterogeneity was observed for LOS, with an I2 statistic > 70%. Consequently, a sensitivity analysis was conducted specifically for LOS (Supplementary Fig. 2). Publication bias was evaluated using funnel plots (Supplementary Fig. 3).
Length of stay (pooled results)
Three studies assessed the impact of PPS on LOS in cholecystectomy cases, with one study examining both LC and open cholecystectomy (OC). A random-effects model was employed for these studies. The analysis indicated that PPS did not significantly alter LOS compared to FFS payment systems (Fig. 2A).
Mortality (pooled results)
Two studies investigated the relationship between the implementation of PPS and postoperative mortality rates following cholecystectomy. The meta-analysis of these studies revealed no significant difference in mortality rates following the adoption of PPS (Fig. 2B).
Complications (non-pooled results)
A study from Taiwan examined two different DRG-based PPS for LC: the DRG-1 group for patients without comorbidities and the DRG-2 group for patients with comorbidities undergoing LC for acute cholecystitis. The findings indicated that DRG categorization effectively differentiated postoperative complications. The DRG-2 group, comprising patients undergoing LC with comorbidities, experienced a higher rate of complications, which consequently resulted in negative financial margins. In another study from the USA evaluating EBP for LC, adult patients were categorized based on the cost profiles of their surgeons: the least expensive (lowest 25%), average (middle 50%), and most expensive (highest 25%). The study revealed significant variation in EBP for LC across different surgeons. The overall complication rate was reported at 11%. Notably, complication rates were significantly higher among patients treated by the most expensive surgeons than those treated by the least expensive (5% for the least expensive, 7% for the average, and 10% for the most expensive surgeons).
Admission (non-pooled results)
Two studies from Israel and Ireland, respectively, indicated a slight increase in cholecystectomy admissions following the transition from FFS to PPS. Conversely, a study from the USA found no significant difference in admission rates when comparing EBP with FFS.
Readmission (non-pooled results)
One study from Israel observed a 4.7% increase in readmission rates for cholecystectomy following the shift from FFS to PPS. Another study from the USA highlighted that under EBP, readmission rates were significantly higher among the most expensive surgeons (14%) than their least expensive counterparts (8%).
Hospital costs (non-pooled results)
One study from Taiwan indicated a significant reduction in costs following the implementation of DRG-case payment for LC. Conversely, there was a significant increase in total costs in the OC group. Another study from Taiwan revealed higher total claims in the DRG-2 patient group, comprising LC patients with comorbidities, compared to the DRG-1 group, comprising LC patients without comorbidities.
Financial margin (non-pooled results)
One study from Israel indicated a decrease in actual hospital income following the introduction of PPS for cholecystectomy, while two other studies from Taiwan reported an increase in financial margins after transitioning to DRG-case payment. Additionally, another study from the USA observed increased financial margins after shifting from FFS to EBP.
The outcomes for cholecystectomy following changes in payment systems are summarized in Table 2. Transitioning from FFS to PPS resulted in no significant changes in LOS and mortality. However, there were minor increases in admission and readmission rates and a decline in financial margins. Specifically, switching from FFS to DRG-case payment demonstrated contrasting outcomes for different cholecystectomy procedures. For LC, this switch led to a reduction in LOS without impacting mortality rates, alongside an increase in financial margins. For OC, there was an observed increase in LOS, which was also accompanied by a rise in financial margins. Moving from FFS to EBP revealed no significant alteration in admission rates but a slight increase in financial margins.
The debate surrounding the impact of PPS on healthcare quality is multifaceted and contentious. Critics argue that by placing financial pressure on hospitals, PPS might compromise the quality of care [40]. Conversely, proponents contend that PPS incentivizes hospitals to enhance care quality as a pathway to cost efficiency [12]. This study represents a pioneering effort to systematically review global literature and synthesize evidence on postoperative outcomes in cholecystectomy post-PPS implementation. Its objective is to provide evidence on the implications of PPS on this standard surgical procedure, offering valuable insights into healthcare policy and economic efficiency in surgical procedures. Our findings reveal that the transition from FFS to PPS, including DRG and EBP, presents a complex picture with both positive and negative outcomes.
Our meta-analysis revealed that the transition to PPS did not significantly decrease LOS in cholecystectomy procedures. This outcome presents a nuanced understanding of the impact of PPS on LOS, diverging from previous reviews that typically suggested a reduction in LOS due to PPS [41-44]. Instead, our results align with other studies that have observed that LOS often remains stable or may even experience a short-term increase following the implementation of DRG-based case payment [45,46]. This variation in outcomes underscores the complexity of healthcare responses to PPS and the necessity for a deeper analysis of the specific circumstances and contexts in which PPS is implemented. For instance, a study in Taiwan observed a notable decrease in LOS from 8 to 5.4 days (p < 0.001) under a DRG-based payment system [35]. However, an Irish study demonstrated only a marginal reduction of 0.1 days post-PPS implementation [39].
The discrepancy in the impact of PPS on LOS may stem from previous reviews’ lack of focus on specific surgeries like cholecystectomy. The complexity of cholecystectomy procedures significantly affects variations in LOS, as more complex cases often necessitate more extended hospital stays [38]. The limited scope of our study, constrained by a few select research papers, may affect the generalizability of our findings. Additionally, while PPS has the potential to reduce inpatient LOS, there is an observed trend where healthcare providers might reallocate services from inpatient to outpatient settings. This shift was evident in a Taiwanese study, where increased outpatient visits were noted in the LC group post-DRG implementation, while outpatient visits decreased but LOS prolonged in the OC group [35]. Such patterns suggest a strategic response by hospitals to financial incentives, possibly leading to a redistribution of healthcare services. Furthermore, hospitals may consciously uphold existing LOS standards to ensure comprehensive patient recovery and maintain high-quality care, particularly for intricate cases. This situation highlights the complex interplay between financial incentives, healthcare service delivery, and the commitment to patient care outcomes, indicating that PPS-induced changes might not straightforwardly result in reduced LOS for surgical procedures like cholecystectomy.
This review revealed no significant impact of PPS on mortality rates in cholecystectomy procedures, echoing findings from a 2010 scoping review [41] that also reported minimal changes in death rates following PPS implementation. In the realm of cholecystectomy, especially since the widespread adoption of laparoscopic techniques in the 1990s, efforts to enhance safety have been paramount. Initiatives such as the “critical view of safety” and the American Gastrointestinal and Endoscopic Surgeons (SAGES) safe cholecystectomy program have significantly reduced major complications like common bile duct injuries, which now occur at a rate of less than 1% [47-52]. This has contributed to maintaining low postoperative mortality rates for cholecystectomy, typically around or below 1% [53]. Hence, it becomes challenging to definitively link PPS to mortality outcomes in cholecystectomy, particularly given the small number of studies focused on this aspect. This scarcity of data underscores the need for more comprehensive, high-quality research to establish a clearer understanding of PPS’s impact on mortality in cholecystectomy patients.
Furthermore, reviewing the impact of PPS on surgical complications, especially in LC for acute cholecystitis, is a pivotal aspect of this study. LC, known for its safety with a low incidence of severe complications [54,55], justifies its inclusion in DRG-based PPS. One Taiwanese study [38] suggests that DRG categorization effectively manages postoperative LOS and complications, particularly for LC patients with comorbidities. Patients classified under the more complex DRG-2 category experienced higher complication rates, underscoring the need for refined approaches in DRG-based PPS to accommodate patients with intricate surgical requirements. The variation in complication rates observed among surgeons under EBP in the USA [37] also highlights the complex dynamics between financial incentives and patient outcomes. It indicates a potential conflict wherein surgeons may face disincentives to treat more complex, high-risk patients due to financial constraints or inadequately structured compensation models. The critical role of comorbidities in clinical risk stratification within the context of PPS is also noteworthy. This suggests that PPS models should consider varying patient complexity, particularly in procedures like LC where comorbidities can significantly impact postoperative resource utilization and outcomes. Moreover, cholecystitis manifests from mild to severe symptoms, making cholecystectomy highly variable in complexity. A nuanced approach within PPS is essential to address the wide-ranging manifestations of cholecystitis and the varying complexity of cholecystectomy procedures. Tailoring DRGs to differentiate between simple and complex procedures could enable more precise reimbursement, reflecting the care intensity required, thus ensuring that hospitals are adequately compensated for complex surgeries without sacrificing care quality. This approach advocates for adaptable payment systems to support high-quality patient care across diverse clinical scenarios.
Our analysis also suggests that PPS should not significantly influence hospital admission and readmission rates, consistent with prior studies [44]. Post-PPS, hospitals often increase outpatient visits to maintain care quality. However, this shift might inadvertently raise readmission rates due to potential postoperative complications in procedures like cholecystectomy. Since readmissions within 30 days are critical indicators of healthcare quality, hospitals, especially in competitive regions, are motivated to minimize these rates. This dynamic underscores the complexity of healthcare management under PPS, where balancing hospital admissions, readmissions, and outpatient care becomes a crucial strategic consideration.
The impact of PPS on hospital costs and financial margins is complex and multifaceted. While one study [35] showed that implementing DRG case payment for LC reduced costs, the opposite trend was observed for OC, potentially due to the varying complexities and resource requirements of these procedures. Regarding financial margins, the response to PPS varied among hospitals: some reported a decline in actual income, whereas others experienced increased margins. This suggests that multiple factors influence PPS’s economic outcomes, including hospital efficiency, patient demographics, and the payment system’s specific structure. The shift to PPS might also incentivize cost-shifting behaviors. Providers motivated by revenue maximization could respond strategically to the financial incentives of PPS, potentially leading to exploitation of the system for monetary gain. This raises questions about the broader implications of PPS on healthcare economics and calls for a deeper investigation into how these payment models influence hospital behaviors, particularly in terms of cost management and financial decision-making. The complexity of these dynamics highlights the need for carefully designed PPS models that align economic incentives with the goals of quality care and efficient resource use.
This study has several limitations. First, the review was based on a relatively small number of studies–only six in total, with just three eligible for meta-analysis. This limited sample size restricts the generalizability of the findings. Second, significant heterogeneity was observed in LOS outcomes, complicating the interpretation of the impact of PPS on this aspect of cholecystectomy care. Third, the reviewed studies spanned a broad temporal range from 2002 to 2023, reflecting potential shifts in healthcare practices and policies that might affect the outcomes over time. Additionally, the variation in healthcare settings and methodologies across the studies could introduce biases or inconsistencies in the results. Fourth, the studies included primarily provided aggregated data on hospital stays, mortality, admissions, readmissions, hospital financial margin, and hospitalization costs without delving into patient-specific details on surgical complications such as biliary tract damage or the necessity to convert laparoscopic cholecystectomies to open surgeries. This gap highlights the need for future research to comprehensively capture and analyze these important surgical outcomes.
Despite these limitations, this study represents the first systematic review examining the impact of PPS on cholecystectomy. It sheds light on how the transition from FFS to PPS does not uniformly influence various aspects such as LOS, mortality, complications, admissions, readmissions, costs, and financial margins on cholecystectomy. The findings underscore the importance of designing and continuously refining PPS models to balance economic efficiency with high-quality patient care. For healthcare decision-makers and practitioners, this study emphasizes the importance of aligning healthcare delivery with economic and patient care goals, especially for prevalent surgeries like cholecystectomy. It underscores the necessity for flexible and detailed healthcare payment reforms tailored to specific surgical procedures and patient demographics.
Supplementary data related to this article can be found at https://doi.org/10.14701/ahbps.24-038.
References
1. Jakovljevic M, Lamnisos D, Westerman R, Chattu VK, Cerda A. Future health spending forecast in leading emerging BRICS markets in 2030: health policy implications. Health Res Policy Syst. 2022; 20:23. DOI: 10.1186/s12961-022-00822-5. PMID: 35183217. PMCID: PMC8857747.
2. Lorenzoni L, Marino A, Morgan D, James C. Health spending projections to 2030: new results based on a revised OECD methodology [Internet]. Available from: https://www.oecd-ilibrary.org/social-issues-migration-health/health-spending-projections-to-2030_5667f23d-en. OECD;2019. cited 2019 May 24.
3. Carrin G, Hanvoravongchai P. 2003; Provider payments and patient charges as policy tools for cost-containment: how successful are they in high-income countries? Hum Resour Health. 1:6. DOI: 10.1186/1478-4491-1-6. PMID: 12914661. PMCID: PMC179884.
4. Mayes R. 2007; The origins, development, and passage of Medicare’s revolutionary prospective payment system. J Hist Med Allied Sci. 62:21–55. DOI: 10.1093/jhmas/jrj038. PMID: 16467485.
5. Jegers M, Kesteloot K, De Graeve D, Gilles W. 2002; A typology for provider payment systems in health care. Health policy. 60:255–273. DOI: 10.1016/S0168-8510(01)00216-0. PMID: 11965334.
6. Ellis RP, Miller M. Carrin G, Buse K, Heggenhougen HK, Quah SR, editors. 2007. Provider payment methods and incentives. Health systems policy, finance, and organization. Academic Press;p. 322–328.
7. Street A, Vitikainen K, Bjorvatn A, Hvenegaard A. Introducing activity-based financing: a review of experience in Australia, Denmark, Norway and Sweden [Internet]. Available from: https://eprints.whiterose.ac.uk/140285. Centre for Health Economics, University of York;2007. cited 2007 Nov 1.
8. Glandon GL, Morrisey MA. 1986; Redefining the hospital-physician relationship under prospective payment. Inquiry. 23:166–175.
9. Cattel D, Eijkenaar F, Schut FT. 2020; Value-based provider payment: towards a theoretically preferred design. Health Econ Policy Law. 15:94–112. DOI: 10.1017/S1744133118000397. PMID: 30259825.
10. Health UDO, Services H. 1983; Medicare program; prospective payments for Medicare inpatient hospital services--HCFA. Interim final rule with comment period. Fed Regist. 48:39752–39890.
11. Preston AM, Chua WF, Neu D. 1997; The diagnosis-related group-prospective payment system and the problem of the government of rationing health care to the elderly. Accounting, Organizations and Society. 22:147–164. DOI: 10.1016/S0361-3682(96)00011-6.
12. Busse R, Geissler A, Aaviksoo A, Cots F, Häkkinen U, Kobel C, et al. 2013; Diagnosis related groups in Europe: moving towards transparency, efficiency, and quality in hospitals? BMJ. 346:f3197. DOI: 10.1136/bmj.f3197. PMID: 23747967.
13. Hodgkin D, Mcguire TG. 1994; Payment levels and hospital response to prospective payment. J Health Econ. 13:1–29. DOI: 10.1016/0167-6296(94)90002-7. PMID: 10134436.
14. Moreno-Serra R, Wagstaff A. 2010; System-wide impacts of hospital payment reforms: evidence from Central and Eastern Europe and Central Asia. J Health Econ. 29:585–602. DOI: 10.1016/j.jhealeco.2010.05.007. PMID: 20566226.
15. Chulis GS. 1991; Assessing Medicare’s prospective payment system for hospitals. Med Care Rev. 48:167–206. DOI: 10.1177/002570879104800203. PMID: 10113662.
16. Congress U. Office of Technology Assessment. Impacts of antibiotic-resistant bacteria. US Government Printing Office;Washington, DC: 1995. Sep. Report No.: OTA-H-629.
17. Yin J, Lurås H, Hagen TP, Dahl FA. 2013; The effect of activity-based financing on hospital length of stay for elderly patients suffering from heart diseases in Norway. BMC Health Serv Res. 13:172. DOI: 10.1186/1472-6963-13-172. PMID: 23651910. PMCID: PMC3651263.
18. Tan SS, Serdén L, Geissler A, van Ineveld M, Redekop K, Heurgren M, et al. Busse R, Geissler A, Quentin W, Wiley M, editors. 2011. DRGs and cost accounting: which is driving which. Diagnosis-related groups in Europe: moving towards transparency, efficiency and quality in hospitals. Open University Press and WHO Regional Office for Europe;p. 59–74.
19. Schuetz P, Albrich WC, Suter I, Hug BL, Christ-Crain M, Holler T, et al. 2011; Quality of care delivered by fee-for-service and DRG hospitals in Switzerland in patients with community-acquired pneumonia. Swiss Med Wkly. 141:w13228. DOI: 10.4414/smw.2011.13228.
20. Carroll NV, Erwin WG. 1990; Effect of the prospective-pricing system on drug use in Pennsylvania long-term-care facilities. Am J Hosp Pharm. 47:2251–2254. DOI: 10.1093/ajhp/47.10.2251.
21. Qian X, Russell LB, Valiyeva E, Miller JE. 2011; "Quicker and sicker" under Medicare’s prospective payment system for hospitals: new evidence on an old issue from a national longitudinal survey. Bull Econ Res. 63:1–27. DOI: 10.1111/j.1467-8586.2010.00369.x. PMID: 21141646.
22. Sutherland JM. Reviewing the potential roles of financial incentives for funding healthcare in Canada: examen du rôle potentiel des incitations financières dans le financement des services de santé au Canada. desLibris;2012.
23. Agresta F, Campanile FC, Vettoretto N, Silecchia G, Bergamini C, Maida P, et al. 2015; Laparoscopic cholecystectomy: consensus conference-based guidelines. Langenbecks Arch Surg. 400:429–453. DOI: 10.1007/s00423-015-1300-4. PMID: 25850631.
24. Fletcher DR. 1995; Laparoscopic cholecystectomy: what national benefits have been achieved and at what cost? Med J Aust. 163:535–538. DOI: 10.5694/j.1326-5377.1995.tb124722.x. PMID: 8538525.
25. Zhao JJ, Syn NL, Chong C, Tan HL, Ng JYX, Yap A, et al. 2021; Comparative outcomes of needlescopic, single-incision laparoscopic, standard laparoscopic, mini-laparotomy, and open cholecystectomy: a systematic review and network meta-analysis of 96 randomized controlled trials with 11,083 patients. Surgery. 170:994–1003. DOI: 10.1016/j.surg.2021.04.004. PMID: 34023139.
26. Weiland DE, Caruso DM, Kassir A, Bay RC, Malone JM. 1997; Using delta/DRG diagrams and decision tree analysis to select a cost-effective surgery for cholecystitis. JSLS. 1:175–180.
27. Page MJ, Mckenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. 2021; The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg. 88:105906. DOI: 10.1016/j.ijsu.2021.105906. PMID: 33789826.
28. Amir-Behghadami M, Janati A. 2020; Population, intervention, comparison, outcomes and study (PICOS) design as a framework to formulate eligibility criteria in systematic reviews. Emerg Med J. 37:387. DOI: 10.1136/emermed-2020-209567. PMID: 32253195.
29. Luo D, Wan X, Liu J, Tong T. 2018; Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. 27:1785–1805. DOI: 10.1177/0962280216669183. PMID: 27683581.
30. Wan X, Wang W, Liu J, Tong T. 2014; Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 14:135. DOI: 10.1186/1471-2288-14-135. PMID: 25524443. PMCID: PMC4383202.
31. Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. 2016; ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 355:i4919. DOI: 10.1136/bmj.i4919. PMID: 27733354. PMCID: PMC5062054.
32. Higgins JP, Thompson SG. 2002; Quantifying heterogeneity in a meta-analysis. Stat Med. 21:1539–1558. DOI: 10.1002/sim.1186. PMID: 12111919.
33. Page MJ, Sterne JA, Higgins JP, Egger M. 2021; Investigating and dealing with publication bias and other reporting biases in meta-analyses of health research: a review. Res Synth Methods. 12:248–259. DOI: 10.1002/jrsm.1468. PMID: 33166064.
34. Shmueli A, Intrator O, Israeli A. 2002; The effects of introducing prospective payments to general hospitals on length of stay, quality of care, and hospitals' income: the early experience of Israel. Soc Sci Med. 55:981–989. DOI: 10.1016/S0277-9536(01)00233-7. PMID: 12220098.
35. Lang HC, Chi C, Liu CM. 2004; Impact of the case payment reimbursement method on the utilization and costs of laparoscopic cholecystectomy. Health Policy. 67:195–206. DOI: 10.1016/S0168-8510(03)00119-2. PMID: 14720637.
36. Chen JL, Chernew ME, Fendrick AM, Thompson JW, Rose S. 2020; Impact of an episode-based payment initiative by commercial payers in Arkansas on procedure volume: an observational study. J Gen Intern Med. 35:578–585. DOI: 10.1007/s11606-019-05318-7. PMID: 31529377. PMCID: PMC7018907.
37. Sheetz KH, Kenney B, Dupree JM, Campbell DA, Englesbe MJ. 2019; Targeting value-driven quality improvement for laparoscopic cholecystectomy in Michigan. Ann Surg. 269:127–132. DOI: 10.1097/SLA.0000000000002438. PMID: 28742681.
38. Wu YT, Lin YN, Cheng CT, Fu CY, Liao CH, Hsieh CH. 2021; Diagnosis-related group (DRG)-based prospective hospital payment system can be well adopted for acute care surgery: Taiwanese experience with acute cholecystitis. World J Surg. 45:1080–1087. DOI: 10.1007/s00268-020-05904-5. PMID: 33454793.
39. Valentelyte G, Keegan C, Sorensen J. 2023; Hospital response to Activity-Based Funding and price incentives: evidence from Ireland. Health Policy. 137:104915. DOI: 10.1016/j.healthpol.2023.104915. PMID: 37741112.
40. Hadley J, Zuckerman S, Feder J. 1989; Profits and fiscal pressure in the prospective payment system: their impacts on hospitals. Inquiry. 354–365.
41. Brügger U, Eichler K. Impact of introducing a DRG reimbursement system in an acute impatient hostpital setting: a literature review. In : HTAi 7th Annual Meeting; 2010 Jun 6-9; Dublin, Ireland.
42. Palmer KS, Agoritsas T, Martin D, Scott T, Mulla SM, Miller AP, et al. 2014; Activity-based funding of hospitals and its impact on mortality, readmission, discharge destination, severity of illness, and volume of care: a systematic review and meta-analysis. PLoS One. 9:e109975. DOI: 10.1371/journal.pone.0109975. PMID: 25347697. PMCID: PMC4210200.
43. Meng Z, Hui W, Cai Y, Liu J, Wu H. 2020; The effects of DRGs-based payment compared with cost-based payment on inpatient healthcare utilization: a systematic review and meta-analysis. Health Policy. 124:359–367. DOI: 10.1016/j.healthpol.2020.01.007. PMID: 32001043.
44. Chen YJ, Zhang XY, Yan JQ, Xue-Tang , Qian MC, Ying XH. 2023; Impact of diagnosis-related groups on inpatient quality of health care: a systematic review and meta-analysis. Inquiry. 60:00469580231167011. DOI: 10.1177/00469580231167011. PMID: 37083281. PMCID: PMC10126696.
45. Thommen D, Weissenberger N, Schuetz P, Mueller B, Reemts C, Holler T, et al. Head-to-head comparison of length of stay, patients' outcome and satisfaction in Switzerland before and after SwissDRG-Implementation in 2012 in 2012: an observational study in two tertiary university centers. Swiss Med Wkly. 2014; 144:w13972. DOI: 10.4414/smw.2014.13972. PMID: 24963880.
46. Jian W, Lu M, Chan KY, Poon AN, Han W, Hu M, et al. 2015; Payment reform pilot in Beijing hospitals reduced expenditures and out-of-pocket payments per admission. Health Aff (Millwood). 34:1745–1752. DOI: 10.1377/hlthaff.2015.0074. PMID: 26438752.
47. Archer SB, Brown DW, Smith CD, Branum GD, Hunter JG. 2001; Bile duct injury during laparoscopic cholecystectomy: results of a national survey. Ann Surg. 234:549–558. DOI: 10.1097/00000658-200110000-00014. PMID: 11573048. PMCID: PMC1422078.
48. Pucci MJ, Brunt ML, Deziel DJ. 2016; Safety first, total cholecystectomy second. J Am Coll Surg. 223:543–544. DOI: 10.1016/j.jamcollsurg.2016.06.005. PMID: 27569667.
49. Pucher PH, Brunt LM, Fanelli RD, Asbun HJ, Aggarwal R. 2015; SAGES expert Delphi consensus: critical factors for safe surgical practice in laparoscopic cholecystectomy. Surg Endosc. 29:3074–3085. DOI: 10.1007/s00464-015-4079-z. PMID: 25669635.
50. Strasberg SM, Brunt ML. 2010; Rationale and use of the critical view of safety in laparoscopic cholecystectomy. J Am Coll Surg. 211:132–138. DOI: 10.1016/j.jamcollsurg.2010.02.053. PMID: 20610259.
51. Strasberg SM, Brunt LM. 2017; The critical view of safety: why it is not the only method of ductal identification within the standard of care in laparoscopic cholecystectomy. Ann Surg. 265:464–465. DOI: 10.1097/SLA.0000000000002054. PMID: 27763898.
52. Sicklick JK, Camp MS, Lillemoe KD, Melton GB, Yeo CJ, Campbell KA, et al. 2005; Surgical management of bile duct injuries sustained during laparoscopic cholecystectomy: perioperative results in 200 patients. Ann Surg. 241:786–792. DOI: 10.1097/01.sla.0000161029.27410.71. PMID: 15849514. PMCID: PMC1357133.
53. Dolan JP, Diggs BS, Sheppard BC, Hunter JG. 2009; The national mortality burden and significant factors associated with open and laparoscopic cholecystectomy: 1997-2006. J Gastrointest Surg. 13:2292–2301. DOI: 10.1007/s11605-009-0988-2. PMID: 19727976.
54. Gurusamy KS, Davidson C, Gluud C, Davidson BR. Early versus delayed laparoscopic cholecystectomy for people with acute cholecystitis. Cochrane Database Sys Rev. 2013; (6):CD005440. DOI: 10.1002/14651858.CD005440.pub3.
55. Song GM, Bian W, Zeng XT, Zhou JG, Luo YQ, Tian X. 2016; Laparoscopic cholecystectomy for acute cholecystitis: early or delayed?: evidence from a systematic review of discordant meta-analyses. Medicine (Baltimore). 95:e3835. DOI: 10.1097/MD.0000000000003835. PMID: 27281088. PMCID: PMC4907666.
Fig. 1
PRISMA flow diagram for data collection. The search returned a total of 106 records, of which 6 studies were included in the review, with 3 being eligible for inclusion in the meta-analysis.
Fig. 2
Forest plots for (A) length of stay and (B) mortality following cholecystectomy. LC, laparoscopic cholecystectomy; OC, open cholecystectomy; MD, mean difference; OR, odds ratio; CI, confidence interval; FFS, fee-for-service; PPS, prospective payment system.
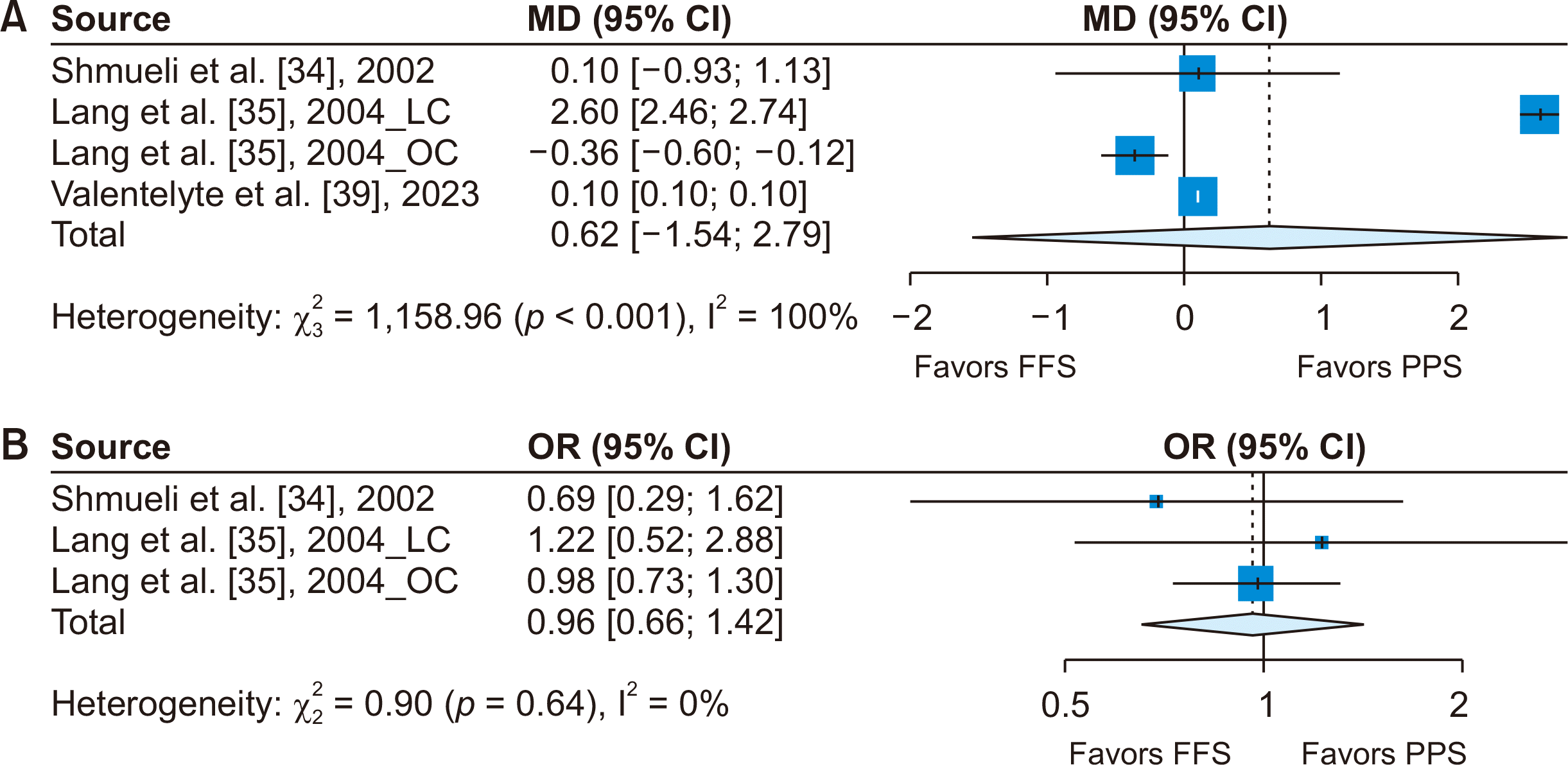
Table 1
Study characteristics (pooled and non-pooled)
| Prospective versus traditional payment system | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Study | Country | Intervention | Data source | Intervention duration | Target population | Pre-implementation payment method |
Post-implementation payment method |
Analytical methods | Outcome |
| Shmueli et al. [34], 2002 | Israel | Fixed PPS for 5 procedures including cholecystectomy | Inpatient hospital records | 1990–1993 | Nationwide study in 4 largest public medical centers in Tel Aviv, Haifa, and Jerusalem | FFS | PPS |
1) Ordinary least squares regression for uneven variance and models the log of hospital stay durations; 2) Poisson regression for LOS; 3) Logit regression evaluated the new PPS system’s effect on readmissions and mortality |
1) LOS 2) Mortality 3) Admission 4) Readmission 5) Hospital financial margin |
| Lang et al. [35], 2004 | Taiwan | DRG-based PPS for laparoscopic and open cholecystectomy | Bureau of National Health Insurance | 1998–1999 | Nationwide (hospital type: not specified) | FFS | DRG |
1) Descriptive analysis (frequency, percentage, mean, standard deviation) for variables; 2) Multiple linear regression to predict total medical expenditures |
1) LOS 2) Mortality 3) Total charge 4) Hospitalization fee |
| Chen et al. [36], 2020 | USA | EBP for 4 procedures including cholecystectomy | Commercial claims data from Truven Health MarketScan Commercial Claims and Encounters database | 2012–2016 | Statewide: Arkansas and control states (states that geographically border Arkansas or are also located in the South Central Census Divisions, excluding Tennessee, Kentucky, and Oklahoma) | FFS | EBP |
1) Difference-in-differences empirical strategy; 2) Analyzed each episode type separately due to variations in clinical areas, payer participation, and different start dates of episode types; 3) Trend analysis for differential pre-EBP trends in outcome variables between Arkansas and control states |
1) The annual rate of procedures; 2) The probability of a beneficiary undergoing that procedure in a given quarter |
| Valentelyte et al. [39], 2023 | Ireland | PPS for minor complexity LC | Inpatient hospital records | 2016–2018 | National Hospital In-Patient Enquiry (HIPE) data and Open Bed Reports data for patients treated in 35 public acute Irish hospitals | FFS | PPS |
1) Quasi-experimental propensity score matching-difference in differences method to evaluate PPS impact on day-case proportion and LOS; 2) Difference-in-differences analysis executed on matched dataset for day-case admissions and LOS; 3) Trend analysis using time trend variables interaction with treatment dummies |
1) LOS 2) Proportion of day-case admissions |
| DRG-based payment | |||||||||
| Study | Country | Intervention | Data source | Intervention duration | Target population | DRG-1 PPS | DRG-2 PPS | Analytical methods | Outcome |
| Wu et al. [38], 2021 | Taiwan | Two DRG-based PPS for LC | Inpatient hospital records | 2015–2016 | Acute cholecystitis patients in Chang Gung Memorial Hospital | DRG-1 PPS: LC without comorbidities/complications | DRG-2 PPS: LC with comorbidities/complications |
1) Patients grouped by DRG with/without major complications/comorbidities and further by acute cholecystitis severity; 2) Descriptive analysis (frequency, percentage, mean, standard deviation) for variables; 3) DRG payment system analyzed and categorized into sectors based on claimed fees |
1) LOS 2) Mortality 3) Total claim, revenue, and financial margin 4) Surgical outcomes and complications |
| EBP stratified by surgeons | |||||||||
| Study | Country | Intervention | Data source | Intervention duration | Target population | EBP | Analytical methods | Outcome | |
| Sheetz et al. [37], 2019 | USA | EBP for LC | Michigan Value Collaborative (MVC) registry: Medicare and a large private payer | 2012–2015 | Statewide: adult patients (≥ 18 yr) with a preoperative diagnosis of symptomatic cholelithiasis or cholecystitis within a Michigan hospital | EBP stratified patients by least expensive (lowest 25%), average (middle 50%), and most expensive (highest 25%) surgeons |
1) Multivariable linear regression models for adjusting payment data, incorporating Hierarchical Condition Categories (HCC) for patient illness differences and yearly payment variations; 2) Reliability adjustments and bootstrapping methods for refining statistical analysis; 3) Sensitivity analyses for specific clinical diagnoses and complication-free patients |
1) Payments included index hospitalization, physician services, readmissions, emergency visits, and post-acute care 2) Complications; 3) Readmission; 4) Use of ambulatory surgical center |
|
Table 2
Summary of outcomes following payment system changes




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download