Abstract
The role of surgical resection in patients with recurrent pancreatic cancer is unclear. We aimed to evaluate the survival outcomes of pancreatic re-resection for locally recurrent pancreatic cancer following index pancreatectomy. A literature search was carried out in CENTRAL, EMBASE, MEDLINE, CINAHL, and Web of Science. Proportion meta-analysis model was constructed to quantify 1 to 5-year survival after pancreatic re-resection for locally recurrent pancreatic cancer. Random-effects modelling was applied to calculate pooled outcome data. Fifteen retrospective studies were included, reporting a total of 250 patients who underwent pancreatic re-resection for locally recurrent pancreatic cancer following their index pancreatectomy. Pancreatic re-resection was associated with 1-year survival 70.6% (95% confidence interval [CI], 65.0−76.2), 2-year survival 38.8% (95% CI, 28.6−49.0), 3-year survival 20.2% (95% CI, 13.8−26.7), and 5-year survival 9.2% (95% CI, 5.5−12.8). The between-study heterogeneity was insignificant in all outcome syntheses. Repeat pancreatectomy for local recurrence of pancreatic cancer in the remnant pancreas following the index pancreatectomy is associated with acceptable overall patient survival. We recommend selective re-resection of such recurrences in younger patients with favorable tumor size and location. Our findings may encourage more robust studies to be conducted in this context to provide stronger evidence.
Pancreatic ductal adenocarcinoma is associated with disappointing prognosis, since the overall 5-year survival rate has been reported to range 5% to 8% [1,2]. Although only surgical resection can potentially cure the disease, the overall survival remains poor, with a reported 5-year survival rate of 12% to 19% after a successful pancreatectomy [3,4]. Such poor prognosis is due to a high rate of cancer recurrence (approximately 80%), even though a complete surgical resection is achieved [5].
Management of pancreatic cancer recurrence has traditionally been limited to best supportive care, with palliative chemotherapy offered to patients who have adequate performance status [6]. Nevertheless, recent evidence indicates that recurrent pancreatic cancer displays diverse behavior according to recurrence timing and location [7]. This includes both local recurrence and distant metastasis. Local recurrence is limited to the remnant pancreas, peripancreatic soft tissue, or locoregional lymph nodes [8,9]. Distant metastasis involves metastasis to a distant organ, distant lymph nodes, and/or the peritoneal space [10]. More recently, an isolated recurrence pattern has been introduced as the first recurrence limited to the remnant pancreas, a single lobe of the lung, a single lobe of the liver, or a single organ, such as the stomach, ovary, adrenal gland, or abdominal wall [11].
The role of surgical resection in patients with recurrent pancreatic cancer is unclear. However, several studies have reported survival benefit of repeat pancreatectomy for selected patients with recurrence in the remnant pancreas after the index pancreatectomy [5,8,9,12-14]. There is no comprehensive synthesis of evidence in this context. Although a meta-analysis of six studies compared the mean patient survival of resection versus no resection of pancreatic cancer recurrence, its findings may be of doubtful merit, considering that the non-resected group did not meet the criteria for resection, indicating that their recurrent disease were more severe and advanced [15]. This consideration subjects the findings of such comparison to significant bias, due to confounding by indication.
The purpose of this study was to conduct a meta-analysis of the best available evidence to evaluate the survival outcomes of pancreatic re-resection for locally recurrent pancreatic cancer following the index pancreatectomy.
We outlined our methodology in a review protocol. The standards of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [16] were respected in the methodology of this study.
All studies investigating the survival outcomes of pancreatic re-resection for locally recurrent pancreatic cancer following the index pancreatectomy were considered.
All male or female adult patients (age more than 18 years) who underwent any type of pancreatectomy for local cancer recurrence following their index pancreatectomy were considered. The index pancreatectomy was defined as any type of pancreatectomy, including open, laparoscopic, or robotic classical pancreaticoduodenectomy (PD), subtotal stomach-preserving PD, pylorus-preserving PD (PPPD), total pancreatectomy (TP), distal pancreatectomy (DP), and central pancreatectomy (CP), for malignant or premalignant pathologies of the pancreas.
The intervention of interest was defined as pancreatectomy for the local recurrence of pancreatic cancer. Other interventions, including exploration, palliative bypass, and other type of organ resection with no need for pancreatic re-resection, were excluded.
We reported 1 to 5-year survival as categorical outcome measures to report the proportion of patients who were alive at a specific point during follow-up.
A strategy for literature search was formulated and run via MEDLINE, CENTRAL, CINAHL, EMBASE, and Web of Sciences (Appendix 1). Moreover, evaluation of the reference lists of the identified studies or reviews was carried out by two independent authors. The literature search was performed on 18th December 2023.
An independent evaluation of the identified articles was performed by two reviewers. When required, their full texts were accessed, and carefully investigated against our inclusion and exclusion criteria. Studies that were deemed eligible were selected for inclusion. Discrepancies during this stage were addressed via detailed discussion among the assessors. If such disagreements remained unresolved, an independent assessor was involved.
A spreadsheet for data extraction was developed, and the information about the included studies, and outcome measures, were collected from all eligible studies by two assessors. Disagreements during this stage were also addressed by consultation with an independent assessor.
The risk of bias assessment of the eligible studies was conducted by two authors using the Institute of Health Economics (IHE) Quality Appraisal Checklist for Case Series Studies [17]. The IHE checklist enables review authors to evaluate a single-arm series in the following aspects: study objective, study design, study population, intervention and co-intervention, outcome measure, statistical analysis, results and conclusions, and competing interests and sources of support. Disagreements following such assessment were addressed via discussion between the assessors. Where disagreements persisted, an additional author was involved.
We used OpenMeta[Analyst] software (Brown School of Public Health) for analysis. The quantitative rates of 1 to 5-year survival were integrated from the included studies. This was followed by calculating an estimate of the overall effect. We used the DerSimonian–Laird random-effects method to determine the weighted summary proportions. Our analysis considered Intention to treat principles when dealing with the extracted data. An individual participant was considered as the unit of analysis. We evaluated heterogeneity through the calculation of I2 using the Cochran Q test (χ2). Heterogeneity was subsequently interpreted whereby 0%−25% was mild, 26%−75% represented moderate heterogeneity, and 76%−100% represented considerable heterogeneity.
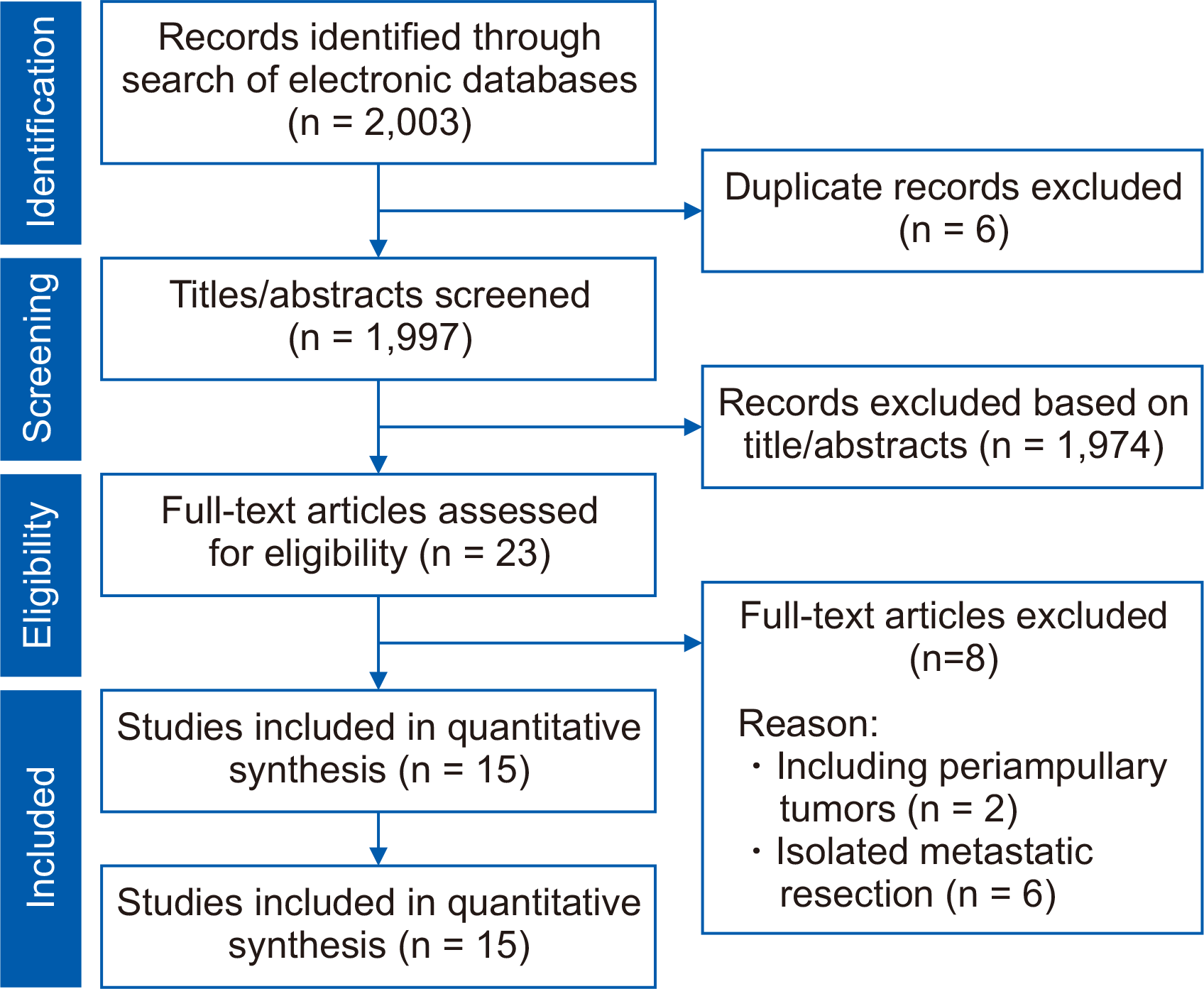
A total of 2,003 articles were detected following the literature search, of which 23 studies were short-listed for further assessment. An additional 8 articles were excluded, as 2 studies reported outcomes of all types of periampullary tumors, and 6 studies reported the outcomes of isolated pancreatic cancer recurrence anywhere, rather than just local recurrence. Finally, 15 retrospectives studies [5,8,9,11-14,18-25] were considered eligible (Fig. 1). The included studies enrolled 250 patients who underwent pancreatectomy for locally recurrent pancreatic cancer following their index pancreatectomy.
Table 1 presents the first author, publication year, country of origin of the corresponding author, journal in which the study was published, sample size, type of index pancreatectomy performed, stage of resected pancreatic cancer, use of adjuvant chemoradiotherapy, and type of re-pancreatectomy.
Of the included studies, 8 reported the median/mean age of the population of interest for this meta-analysis, which ranged 56 to 70 years. Considering the studies that reported the gender of their included patients, 56% of the patients were male, while the remaining 44% were female patients. The pathological staging of the primarily resected pancreatic cancer varied among the included studies, with stage II being the most common, followed by stage I, III, and a very few cases of stage IV disease. Ten studies reported the nature of the index pancreatectomies, which included 61.4% PD, 38.0% DP, and 0.6% CP. The adjuvant chemoradiotherapy was considered for 65.1% of the patients, while the remaining 34.9% did not receive any form of adjuvant treatment. Twelve studies provided information about the nature of pancreatic re-resections, which included 26.8% PD, 36.6% DP, 20.9% TP, 3.1% CP, and 12.6% unspecified.
Fig. 2 outlines the results of risk of bias assessment of all included studies. The risk of bias associated with the study population was low in 8 studies, unclear in 4 studies, and high in 3 studies. The risk of bias associated with intervention and co-intervention was low in 12 studies, and unclear in 3 studies. The risk of bias associated with outcome measure was low in 14 studies, but unclear in one study. The risk of bias associated with study objective, study design, statistical analysis, results, and conclusions, or competing interests and sources of support, were low in all the included studies.
Fig. 3 presents the results of the outcome syntheses.
1-year survival
Fourteen studies with a pooled population size of 244 patients were included in the analysis, which demonstrated that the 1-year survival rate after pancreatic re-resection was 70.6% (95% confidence interval [CI], 65.0−76.2) (Fig. 3A). A low level of heterogeneity was detected (I2 = 0%, p = 0.561).
2-year survival
Fourteen studies with a pooled population size of 244 patients were included in the analysis, which demonstrated that the 2-year survival rate after pancreatic re-resection was 38.8% (95% CI, 28.6−49.0) (Fig. 3B). A moderate level of heterogeneity was detected (I2 = 60.49%, p = 0.002).
3-year survival
Thirteen studies with a pooled population size of 238 patients were included in the analysis, which demonstrated that the 3-year survival rate after pancreatic re-resection was 20.2% (95% CI, 13.8−26.7) (Fig. 3C). A moderate level of heterogeneity was detected (I2 = 29.8%, p = 0.145).
5-year survival
Thirteen studies with a pooled population size of 238 patients were included in the analysis, which demonstrated that the 5-year survival rate after pancreatic re-resection was 9.2% (95% CI, 5.5−12.8) (Fig. 3D). The between-study heterogeneity was low (I2 = 3.03%, p = 0.416).
There has been growing evidence in favor of repeat pancreatectomy for isolated recurrent pancreatic cancer in the remnant pancreas. A comprehensive systematic review and meta-analysis was conducted to evaluate the survival outcomes of pancreatic re-resection for locally recurrent pancreatic cancer following the index pancreatectomy. We identified 15 observational studies [5,8,9,11-14,18-25] reporting a total of 250 patients who underwent re-resection of the remnant pancreas for locally recurrent pancreatic cancer. The subsequent analyses demonstrated that pancreatic re-resection was associated with 1-year survival of 70.6%, 2-year survival of 38.8%, 3-year survival of 20.2%, and 5-year survival of 9.2%. The degree of heterogeneity among the analyzed studies was insignificant in the analysis of all evaluated outcomes.
Surgical resection of locally recurrent pancreatic cancer has been considered an attractive, albeit infrequent, approach to be included in a multimodal treatment strategy alongside other systemic treatments [26]. However, in the context of the management of isolated local pancreatic cancer recurrence, an established treatment strategy or a definitive guideline is lacking. Similarly, no comprehensive evidence synthesis exists in the literature. Serafini et al. [15] conducted a meta-analysis of six studies to compare the outcomes of surgical resection and non-surgical treatments of recurrent pancreatic cancer, and reported that the overall survival and post-recurrence survival were significantly longer in the re-resected group. However, the patients in the non-resected group did not meet the criteria for any surgical resection, as their disease was too advanced, or had metastasis. For example, in both Hashimoto et al. [12] and Strobel et al. [20] (both included in the pooled analysis by Serafini et al. [15]), the patients who did not have resection were deemed to have unresectable disease, due to having distant metastasis or arterial involvement. Therefore, the findings of such comparison should be of doubtful merit due to the existence of significant bias, due to confounding by indication. It is important to highlight that a repeat pancreatectomy may be beneficial for a selected sub-group of patients who have a sufficient performance status and have recurrence limited to the remnant pancreas without any major vessel invasion, and with no active neural invasion, considering that in such cases, an R0 resection is relatively more likely to be achieved [27]. In fact, after a repeat pancreatectomy, the median survival seems to be relatively more favorable at 25−44 months in patients with recurrence limited to the pancreatic remnant [27]. Yamada et al. [24] identified the most favorable outcomes for re-resection in patients aged < 65 years with tumor size < 20 mm at least 10 mm away from the pancreatic stump. Whilst stratification of outcomes with respect to age, tumor size, or tumor distance from the pancreatic stump would have therefore been important, we were unable to do so, due to inconsistent reporting within the included studies.
The most performed type of index pancreatectomy in the included patients was PD (61.4%), followed by DP (38.0%). This may explain the relatively higher rate of distal/total remnant pancreatectomy (57.8%) as the most common type of repeat pancreatectomy procedure, when compared to PD (26.8%). Although our findings suggest acceptable survival rates of repeat pancreatectomy for local recurrence, it is worth highlighting that completion pancreatectomy is associated with endocrine and exocrine pancreatic insufficiency, with consequences that include diabetes and malabsorption. This also influences chemotherapy tolerance, whereby patients are more likely to suffer from diarrhea, hypoglycemia, and weight loss [22]. We were not able to evaluate such sequelae of repeat pancreatectomy in this meta-analysis. Furthermore, objective assessment of quality of life in such patient group deserves the attention of future research in this context.
Interestingly, most of the included studies were conducted in Asian countries, followed by the United States and Germany. To the best of our knowledge, there is no published case of repeat pancreatectomy for the local recurrence of pancreatic cancer in the United Kingdom (UK). Nevertheless, absence of evidence is not evidence of absence. Although, as a UK based center, we had an experience in performing completion pancreatectomy for the local recurrence of pancreatic cancer following a PPPD with survival benefit for the treated patient, such experience was an isolated episode, which has never been translated into common practice. We believe there is a need for an established evidence-based guideline in this context. The findings of this meta-analysis can be utilized to design and conduct better quality studies to evaluate comparative outcomes of surgical resection versus non-surgical management of isolated locally recurrent pancreatic cancer in patients with comparable characteristics, to evaluate the real survival benefit of repeat pancreatectomy in such a patient group.
The available evidence is heterogenous about the use of chemotherapy before and after the re-resection of pancreatic cancer recurrence. Local recurrence of pancreatic cancer following the index pancreatectomy should be considered as an indication for systemic chemotherapy due to the high possibility of the presence of micrometastasis [28,29]. Whether or not the newly diagnosed cancer is a true local recurrence or a new primary lesion, neoadjuvant chemotherapy before re-resection may have some survival advantages. Adjuvant chemotherapy following pancreatic re-resection has also been demonstrated to significantly improve median survival in such a patients’ group [14]. However, the certainty and level of evidence remains low.
Readers of this study should consider its recognized limitations. The included studies had a retrospective nature, indicating that our results are at risk of bias associated with such study designs. This, together with the unclear or high risk of bias associated with the study population in nearly half of the included studies, can negatively impact the robustness of our findings. Although conducting a high-quality prospective comparative study in this context is challenging, the establishment of a best available evidence-based guideline in this context can subsequently encourage more frequent pancreatic re-resection for locally recurrent pancreatic cancer in selected patients, which can provide the opportunity to conduct higher quality studies with larger sample sizes in the future. The current meta-analysis is a single-arm meta-analysis, and does not provide any information about the potential advantages of repeat pancreatectomy over the non-surgical management of the local recurrence of pancreatic cancer in patients with homogenous disease characteristics. The sample sizes of the included studies were very small. We were unable to conduct our analyses with respect to the important determinants of outcomes, including tumor size, stage of the primary cancer, use of adjuvant chemotherapy after the index pancreatectomy, co-morbidities, or the type of index and repeat pancreatectomies.
Considering our literature review and the findings of our meta-analysis, we encourage future studies:
• To consider the comparative outcomes of repeat pancreatectomy over the non-surgical management of the local recurrence of pancreatic cancer in patients with homogenous disease characteristics.
• To consider evaluation of the quality of life in patients undergoing pancreatic re-resection for locally recurrent pancreatic cancer.
Repeat pancreatectomy for the local recurrence of pancreatic cancer in the remnant pancreas following the index pancreatectomy is associated with acceptable overall patient survival. The best available evidence is limited to small-sized retrospective single-arm studies, with the inherited risk of bias associated with their included populations. We recommend the selective re-resection of such recurrences in younger patients with favorable tumor size and location. Our findings may encourage more robust studies to be conducted in this context to provide stronger evidence.
References
1. Siegel R, Naishadham D, Jemal A. 2013; Cancer statistics, 2013. CA Cancer J Clin. 63:11–30. DOI: 10.3322/caac.21166. PMID: 23335087.
2. Tempero MA, Malafa MP, Al-Hawary M, Asbun H, Bain A, Behrman SW, et al. 2017; Pancreatic adenocarcinoma, version 2.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 15:1028–1061. DOI: 10.6004/jnccn.2017.0131. PMID: 28784865.
3. Ferrone CR, Pieretti-Vanmarcke R, Bloom JP, Zheng H, Szymonifka J, Wargo JA, et al. 2012; Pancreatic ductal adenocarcinoma: long-term survival does not equal cure. Surgery. 152(3 Suppl 1):S43–S49. DOI: 10.1016/j.surg.2012.05.020. PMID: 22763261. PMCID: PMC3806092.
4. Ferrone CR, Brennan MF, Gonen M, Coit DG, Fong Y, Chung S, et al. 2008; Pancreatic adenocarcinoma: the actual 5-year survivors. J Gastrointest Surg. 12:701–706. DOI: 10.1007/s11605-007-0384-8. PMID: 18027062.
5. Suzuki S, Furukawa T, Oshima N, Izumo W, Shimizu K, Yamamoto M. 2016; Original scientific reports: clinicopathological findings of remnant pancreatic cancers in survivors following curative resections of pancreatic cancers. World J Surg. 40:974–981. DOI: 10.1007/s00268-015-3353-5. PMID: 26589594. PMCID: PMC4767846.
6. Groot VP, Daamen LA, Hagendoorn J, Borel Rinkes IHM, Busch OR, van Santvoort HC, et al. 2017; Current strategies for detection and treatment of recurrence of pancreatic ductal adenocarcinoma after resection: a nationwide survey. Pancreas. 46:e73–e75. DOI: 10.1097/MPA.0000000000000899. PMID: 28902799.
7. Groot VP, Blair AB, Gemenetzis G, Ding D, Burkhart RA, van Oosten AF, et al. 2019; Isolated pulmonary recurrence after resection of pancreatic cancer: the effect of patient factors and treatment modalities on survival. HPB (Oxford). 21:998–1008. DOI: 10.1016/j.hpb.2018.12.002. PMID: 30777697.
8. Thomas RM, Truty MJ, Nogueras-Gonzalez GM, Fleming JB, Vauthey JN, Pisters PW, et al. 2012; Selective reoperation for locally recurrent or metastatic pancreatic ductal adenocarcinoma following primary pancreatic resection. J Gastrointest Surg. 16:1696–1704. DOI: 10.1007/s11605-012-1912-8. PMID: 22644446. PMCID: PMC3884897.
9. Miyazaki M, Yoshitomi H, Shimizu H, Ohtsuka M, Yoshidome H, Furukawa K, et al. 2014; Repeat pancreatectomy for pancreatic ductal cancer recurrence in the remnant pancreas after initial pancreatectomy: is it worthwhile? Surgery. 155:58–66. DOI: 10.1016/j.surg.2013.06.050. PMID: 24238124.
10. Sugimoto M, Mitsunaga S, Yoshikawa K, Kato Y, Gotohda N, Takahashi S, et al. 2014; Prognostic impact of M2 macrophages at neural invasion in patients with invasive ductal carcinoma of the pancreas. Eur J Cancer. 50:1900–1908. DOI: 10.1016/j.ejca.2014.04.010. PMID: 24835032.
11. Kim YI, Song KB, Lee YJ, Park KM, Hwang DW, Lee JH, et al. 2019; Management of isolated recurrence after surgery for pancreatic adenocarcinoma. Br J Surg. 106:898–909. DOI: 10.1002/bjs.11144. PMID: 31162655.
12. Hashimoto D, Chikamoto A, Ohmuraya M, Sakata K, Miyake K, Kuroki H, et al. 2014; Pancreatic cancer in the remnant pancreas following primary pancreatic resection. Surg Today. 44:1313–1320. DOI: 10.1007/s00595-013-0708-0. PMID: 23975591.
13. Shima Y, Okabayashi T, Kozuki A, Sumiyoshi T, Tokumaru T, Saisaka Y, et al. 2015; Completion pancreatectomy for recurrent pancreatic cancer in the remnant pancreas: report of six cases and a review of the literature. Langenbecks Arch Surg. 400:973–978. DOI: 10.1007/s00423-015-1355-2. PMID: 26545606.
14. Nakayama Y, Sugimoto M, Gotohda N, Konishi M, Takahashi S. 2018; Efficacy of completion pancreatectomy for recurrence of adenocarcinoma in the remnant pancreas. J Surg Res. 221:15–23. DOI: 10.1016/j.jss.2017.07.016. PMID: 29229121.
15. Serafini S, Sperti C, Friziero A, Brazzale AR, Buratin A, Ponzoni A, et al. 2021; Systematic review and meta-analysis of surgical treatment for isolated local recurrence of pancreatic cancer. Cancers (Basel). 13:1277. DOI: 10.3390/cancers13061277. PMID: 33805716. PMCID: PMC7998253.
16. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. 2009; The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 339:b2700. DOI: 10.1136/bmj.b2700. PMID: 19622552. PMCID: PMC2714672.
17. Institute of Health Economics (IHE). Quality appraisal of case series studies checklist [Internet]. Available from: http://www.ihe.ca/research-programs/rmd/cssqac/cssqac-about. Institute of Health Economics;2014. cited 2023 Dec 19.
18. Kleeff J, Reiser C, Hinz U, Bachmann J, Debus J, Jaeger D, et al. 2007; Surgery for recurrent pancreatic ductal adenocarcinoma. Ann Surg. 245:566–572. DOI: 10.1097/01.sla.0000245845.06772.7d. PMID: 17414605. PMCID: PMC1877037.
19. Lavu H, Nowcid LJ, Klinge MJ, Mahendraraj K, Grenda DR, Sauter PK, et al. 2011; Reoperative completion pancreatectomy for suspected malignant disease of the pancreas. J Surg Res. 170:89–95. DOI: 10.1016/j.jss.2011.04.050. PMID: 21696765.
20. Strobel O, Hartwig W, Hackert T, Hinz U, Berens V, Grenacher L, et al. 2013; Re-resection for isolated local recurrence of pancreatic cancer is feasible, safe, and associated with encouraging survival. Ann Surg Oncol. 20:964–972. DOI: 10.1245/s10434-012-2762-z. PMID: 23233235.
21. Boone BA, Zeh HJ, Mock BK, Johnson PJ, Dvorchik I, Lee K, et al. 2014; Resection of isolated local and metastatic recurrence in periampullary adenocarcinoma. HPB (Oxford). 16:197–203. DOI: 10.1111/hpb.12119. PMID: 23601033. PMCID: PMC3945844.
22. Ishida J, Toyama H, Matsumoto I, Asari S, Goto T, Terai S, et al. 2016; Second primary pancreatic ductal carcinoma in the remnant pancreas after pancreatectomy for pancreatic ductal carcinoma: high cumulative incidence rates at 5 years after pancreatectomy. Pancreatology. 16:615–620. DOI: 10.1016/j.pan.2016.05.003. PMID: 27237099.
23. Chang SC, Hsu CP, Tsai CY, Liu YY, Liu KH, Hsu JT, et al. 2016; Selective reoperation after primary resection as a feasible and safe treatment strategy for recurrent pancreatic cancer. Medicine (Baltimore). 95:e4191. DOI: 10.1097/MD.0000000000004191. PMID: 27472688. PMCID: PMC5265825.
24. Yamada S, Kobayashi A, Nakamori S, Baba H, Yamamoto M, Yamaue H, et al. 2018; Resection for recurrent pancreatic cancer in the remnant pancreas after pancreatectomy is clinically promising: results of a project study for pancreatic surgery by the Japanese Society of Hepato-Biliary-Pancreatic Surgery. Surgery. 164:1049–1056. DOI: 10.1016/j.surg.2018.05.050. PMID: 30068484.
25. Lee B, Han HS, Lee JS, Yoon YS. 2021; Surgical resection or ablation for recurrent pancreatic ductal adenocarcinoma: an analysis of oncologic outcomes according to the recurrence type. Ann Surg Open. 2:e096. DOI: 10.1097/AS9.0000000000000096. PMID: 37635830. PMCID: PMC10455453.
26. Hidalgo M. 2010; Pancreatic cancer. N Engl J Med. 362:1605–1617. Erratum in: N Engl J Med 2010;363:298. DOI: 10.1056/NEJMra0901557. PMID: 20427809.
27. Okusaka T. 2022; Treatment for postoperative recurrence of pancreatic cancer: a narrative review. Chin Clin Oncol. 11:19. DOI: 10.21037/cco-21-87. PMID: 35695055.
28. Sohal DP, Walsh RM, Ramanathan RK, Khorana AA. 2014; Pancreatic adenocarcinoma: treating a systemic disease with systemic therapy. J Natl Cancer Inst. 106:dju011. DOI: 10.1093/jnci/dju011. PMID: 24563516.
29. Sohal DPS, Kennedy EB, Khorana A, Copur MS, Crane CH, Garrido-Laguna I, et al. 2018; Metastatic pancreatic cancer: ASCO clinical practice guideline update. J Clin Oncol. 36:2545–2556. DOI: 10.1200/JCO.2018.78.9636. PMID: 29791286. PMCID: PMC7504972.
Fig. 2
Risk of bias summary and graph showing the authors’ judgments about each risk of the bias item.
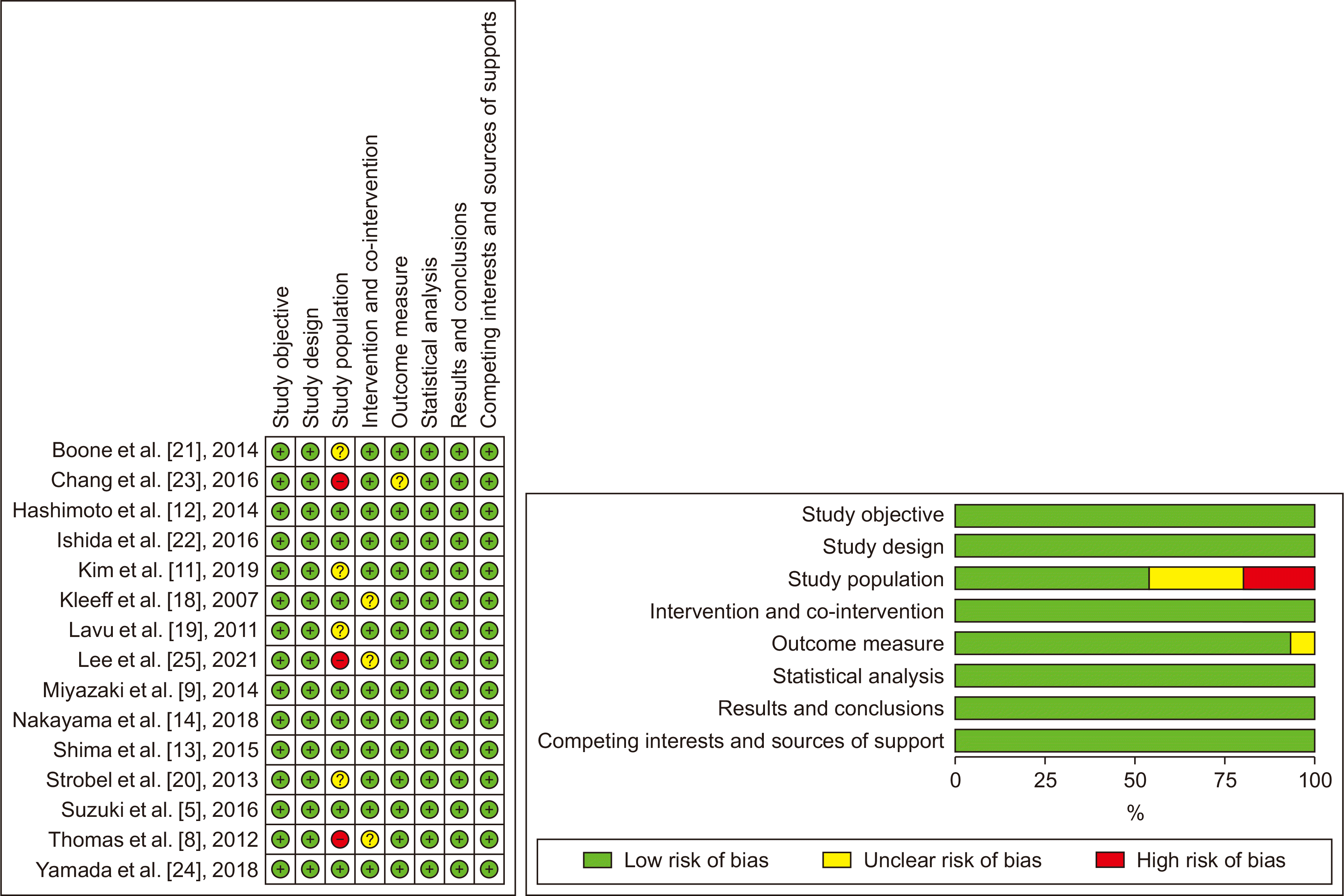
Fig. 3
Forest plots for proportion meta-analysis of the survival outcomes after re-resection of the local recurrence of pancreatic cancer: (A) 1-year survival, (B) 2-year survival, (C) 3-year survival, and (D) 5-year survival. CI, confidence interval; Ev/Trt, event/treatment.
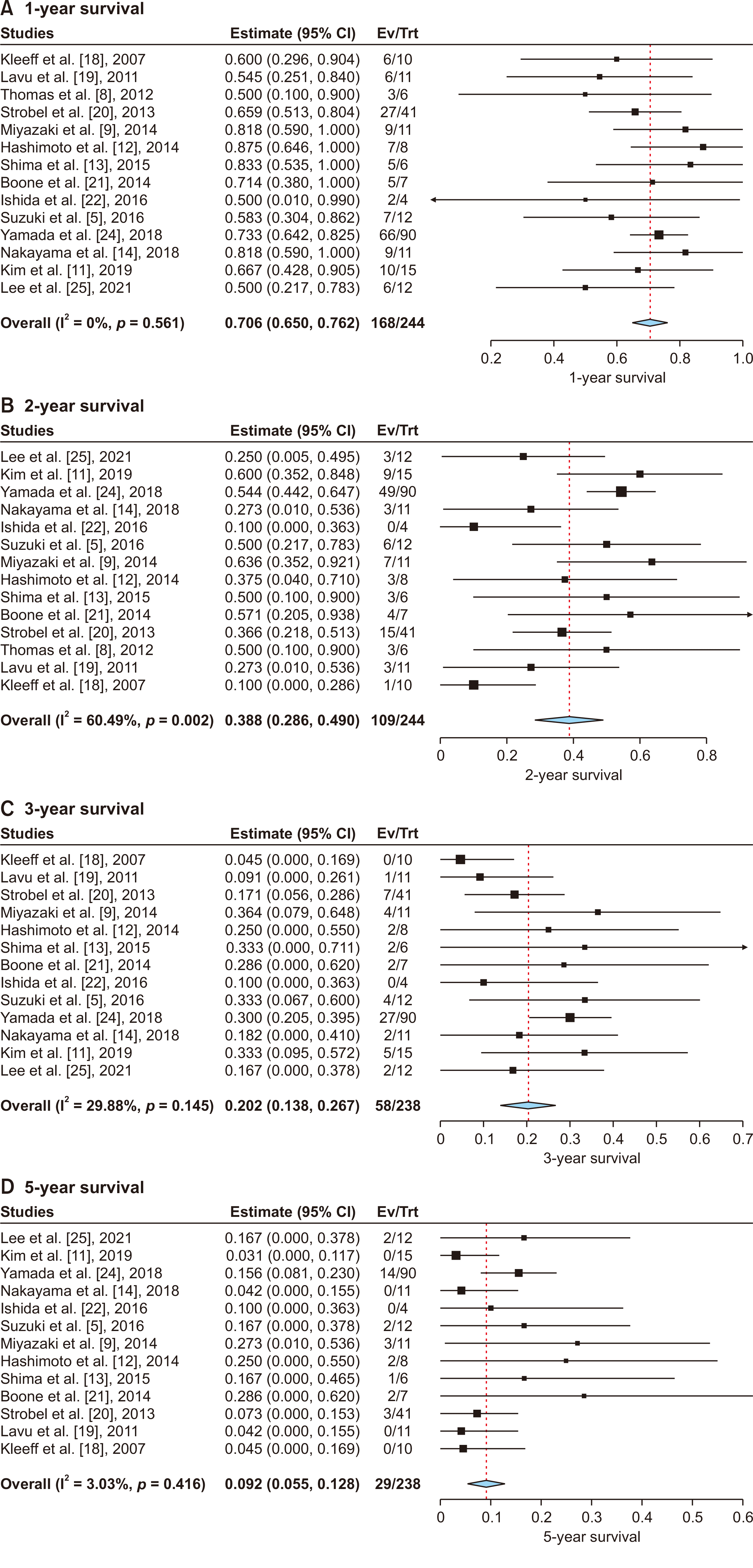
Table 1
Study-related data and baseline demographic and clinical characteristics of the included patients
| Author | Year | Country | Journal | Sample size | Age (yr) | Sex | TNM stage | Index operation | Adjuvant CRT | Re-operation for local recurrence |
|---|---|---|---|---|---|---|---|---|---|---|
| Kleeff et al. [18] | 2007 | Germany | Annals of Surgery | 10 | NR | NR | II: 10 |
PD: 7 DP: 3 |
Yes: 4, No: 6 | Not specified |
| Lavu et al. [19] | 2011 | USA | Journal of Surgical Research | 11 | 69 (31–82) | 6M/5F | NR |
PD: 7 DP: 4 |
Yes: 8, No: 3 |
PD: 4 DP: 5 DP + adrenalectomy: 1 DP + subtotal gastrectomy: 1 |
| Thomas et al. [8] | 2012 | USA | Journal of Gastrointenstinal Surgery | 6 | NR | NR | I or II: 6 | NR | NR | NR |
| Strobel et al. [20] | 2013 | Germany | Annals of Surgical Oncology | 41 | NR | NR | NR | NR | NR |
PD: 9 DP: 10, SPR: 5 Pancreatic resection + other organs: 14 Other: 3 |
| Miyazak et al. [9] | 2014 | Japan | Surgery | 11 | 67 (60–80) | 6M/5F |
I: 1 II: 8 III:1 IV: 1 |
PD: 4 PD + PV: 2 PD + HR: 1 DP: 4 |
Yes: 6, No: 5 |
TP: 8 TP + PV: 1 TP + gastrectomy: 1 DP: 1 |
| Hashimoto et al. [12] | 2014 | Japan | Surgery Today | 8 | 70 (55–80) | 4M/4F |
I: 3 II: 5 |
PD: 4 DP: 4 |
Yes: 3, No: 5 |
TP: 6 TP + PV: 1 CP: 1 |
| Shima et al. [13] | 2015 | Japan | Langenbeck’s Archives of Surgery | 6 | 66 (52–82) | 3M/3F |
I: 1 II: 5 |
PD: 3 PD + PV: 1 DP: 2 |
Yes: 1, No: 6 |
TP: 3 TP + PV: 2 DP: 1 |
| Boone et al. [21] | 2014 | USA | HPB | 7 | NR | NR | II: 7 |
PD: 4 PD + PV: 1 DP: 2 |
NR |
TP: 2 DP: 2 Resection of pancreatic bed mass: 1 Other: 2 |
| Ishida et al. [22] | 2016 | Japan | Pancreatology | 4 | 56 (50–62) | 2M/2F |
I: 2 II: 2 |
PD: 2 DP: 2 |
Yes: 4, No: 0 | TP: 4 |
| Chang et al. [23] | 2016 | Taiwan | Medicine | 6 | NR | NR |
I: 6 II: 8 |
NR | NR |
PD: 1 TP: 1 DP: 1 Other: 3 |
| Suzuki et al. [5] | 2016 | Japan | World Journal of Surgery | 12 | 59.5 (55–69) | 6M/6F |
I: 2 II: 9 IV: 1 |
PD: 3 PD + PV: 2 DPPHR: 1 DP: 6 |
Yes: 6, No: 6 |
TP: 8 TP + PV: 2 DP: 1 DPPHR: 1 |
| Yamada et al. [24] | 2018 | Japan | Surgery | 90 | 64.4 ± 8.3 | 50M/40F |
0: 1 I: 26 II: 58 III: 4 |
PD: 53 DP: 36 CP: 1 |
Yes: 63, No: 27 |
PD: 37 DP: 53 |
| Nakayama et al. [14] | 2018 | Japan | Journal of Surgical Research | 11 | 68 (37–73) | 8M/3F |
I + II: 3 III + IV: 8 |
PD: 8 DP: 3 |
Yes: 10, No: 1 | TP: 11 |
| Kim et al. [11] | 2019 | South Korea | British Journal of Surgery | 15 | NR | NR | NR | NR | NR | TP or DP: 15 |
| Lee et al. [25] | 2021 | South Korea | Annals of Surgery Open | 12 | NR | NR | NR | NR | NR | NR |
TNM, tumor, node, metastasis; CRT, chemoradiotherapy; NR, not reported; PD, pancreaticoduodenectomy; DP, distal pancreatectomy; SPR, segmental pancreatic resection; TP, total pancreatectomy; PV, portal vein resection; HR, hepatic resection; CP, central pancreatectomy; DPPHR, duodenum preserving pancreas head resection.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download