Abstract
Cardiovascular disease management has made significant progress with lipid-lowering interventions, primarily statin therapy. However, statins' side effects, combined with their variable efficacy, have sparked interest in alternative treatments, particularly proprotein convertase subtilisin/kexin type 9 (PCSK9) monoclonal antibodies. These biologics, approved by the US Food and Drug Administration and the European Medicines Agency, have shown a significant impact on lipid levels, particularly low-density lipoprotein cholesterol (LDL-C), resulting in a 50% to 60% reduction. Despite the benefits of PCSK9 inhibitors, the guidelines for their use differ, with specific thresholds determining eligibility. The National Institute for Health and Care Excellence recommends starting PCSK9 therapy for patients with LDL-C levels above 3.5 mmol/L and lipid levels above 5.0 mmol/L who do not have cardiovascular disease. This rigid framework, while cost-effective, may exclude a subset of patients who do not meet these criteria despite having a high cardiovascular risk. The limited scope of these guidelines presents a challenge for specialists managing patients excluded as a result of LDL-C levels that fall just below the threshold but still show signs of significant cardiovascular risk. Recent audits revealed that a significant proportion of patients fall into this grey area, emphasizing the importance of re-evaluating LDL targets for PCSK9 inhibitor initiation. Biological variations, pharmacogenomics, and other factors all contribute to this challenge, highlighting the importance of personalized medicine.
Patients with cardiovascular diseases (CVDs) have significantly benefited for decades from lipid-lowering drugs, with reductions in overall morbidity and mortality. Notably, the Framingham Heart Study [1] and Münster Heart Study [2] were among the first epidemiological studies to recognize the detrimental effects of lipids on the development of CVD around two decades ago. More than a century earlier, Anitschow had already observed signs of this association between lipids and CVD in histological samples of atheromatous plaque [3–5]. These observations paved the way for a deeper understanding of the adverse effects of lipids and spurred the development of lipid-lowering drugs, significantly reducing the risk of coronary heart disease.
Presently, seven million people in the United Kingdom are on statins [6]. Over the past 30 years, statins have proven effective in reducing abnormal lipid profiles, particularly by lowering low-density lipoprotein cholesterol (LDL-C) levels. However, recent media coverage has highlighted the adverse effects associated with statins, notably myopathy, in certain patient subgroups [7]. These individuals are identified as having variants in the gene in the solute carrier organic anion transporter family member 1B1 (SLCO1B1) gene that predispose them to statin-related myopathy. However, Hirsh et al. [8] pointed out shortcomings in the primary healthcare system, noting that physicians often either fail to identify patients who would benefit from statin therapy or encounter resistance from patients when suggesting statins as first-line treatment.
Patients who are intolerant to or have not responded to all lines of lipid-lowering drugs are advised to consider proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors as a final option in their lipid management strategy. This pivotal discovery was made by Abifadel et al. [9], who found that mutations in PCSK9 were responsible for autosomal dominant familial hypercholesterolemia. The US Food and Drug Administration (FDA) and the European Medicines Agency have approved two monoclonal antibodies targeting PCSK9: Praluent (alirocumab, developed by Sanofi-Aventis and Regeneron Pharmaceuticals Inc) and Repatha (evolocumab, developed by Amgen Inc). These medications have been authorized by the FDA for use alongside dietary modifications.
The introduction of PCSK9 inhibitors in 2015 marked a significant advancement in lipid clinics nationwide, leading to positive outcomes for patients who began receiving these biologics. Both physicians and patients quickly embraced these treatments, particularly appreciating their convenience and the option of biweekly or monthly dosages.
PCSK9 is an enzyme encoded on chromosome 1 that plays a crucial role in cholesterol metabolism through its regulation of LDL receptors. The activity of PCSK9 can alter blood levels of LDL-C by modulating the LDL receptor. PCSK9 targets the receptors on LDL molecules and facilitates their degradation within the liver. It has been identified as a genetic contributor to familial hypercholesterolemia due to its involvement in the degradation and recycling of LDL-C receptor levels. Recently, PCSK9 has been the focus of new lipid-lowering drug developments, specifically in the form of monoclonal antibodies (mAbs) that inhibit PCSK9's activity. These mAbs work by binding to PCSK9, which allows LDL receptors to be freed and relocated to the surface of liver cells. There, they can capture excess LDL from the bloodstream and transport it into hepatocytes for processing and elimination.
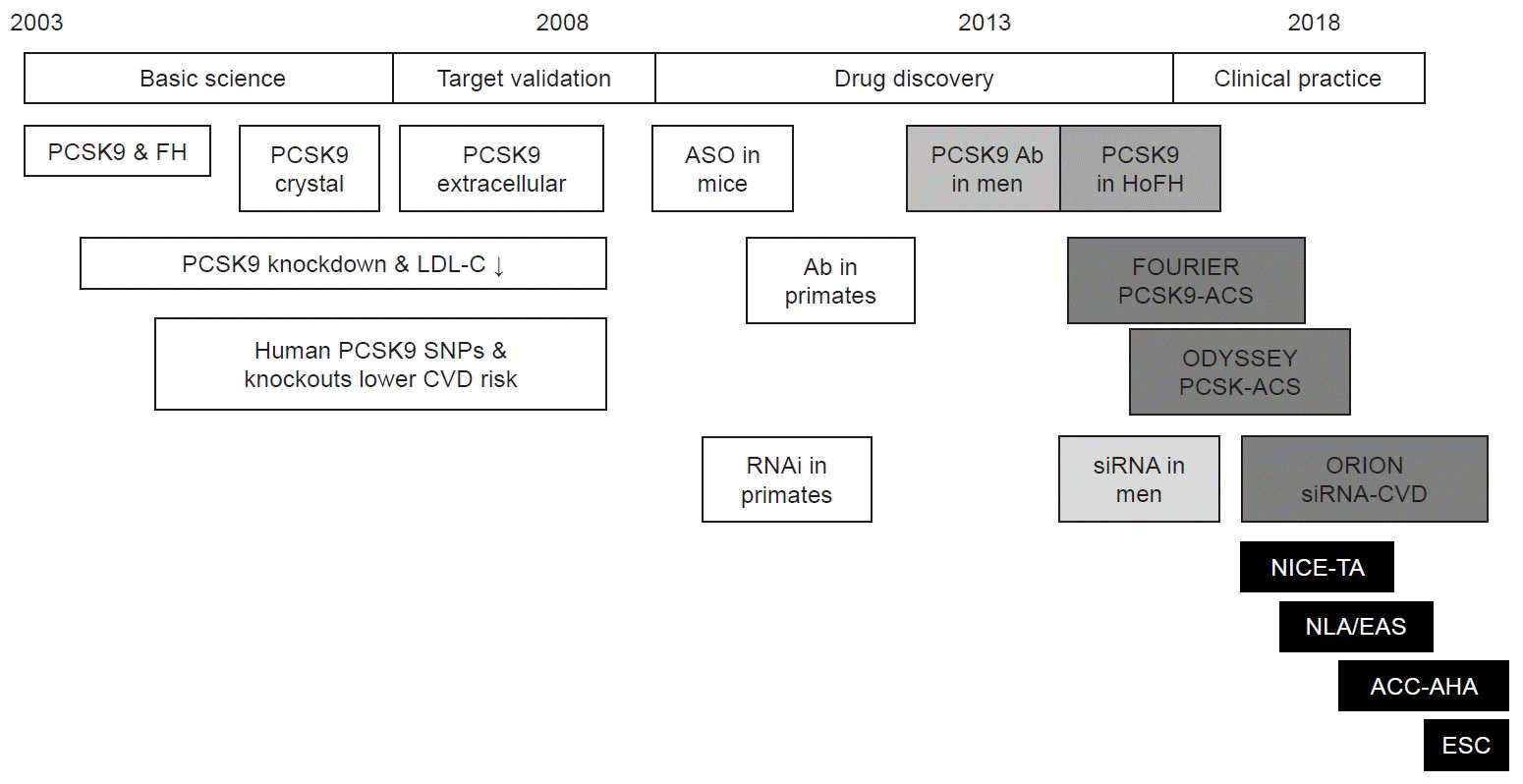
The introduction of PCSK9 mAbs in secondary healthcare settings is of paramount importance, as evidenced by its positive outcome data (Fig. 1) [10]. It significantly reduces lipid levels in patients, whether they have comorbidities or not, lowering their LDL-C by 50% to 60%. Guidelines have been developed to assist healthcare providers in secondary care settings in treating patients with dyslipidaemia, regardless of whether the causes are primary or secondary. The National Institute for Health and Care Excellence (NICE) advises against prescribing alirocumab to patients who do not have CVD [11]. However, individuals with lipid levels above 193 mg/dL (5.0 mmol/L) and no CVD may be considered for PCSK9 inhibitors. This represents a logical, stepwise approach to managing lipid disorders, aiming to optimize the use of common lipid-lowering drugs before resorting to PCSK9 inhibitors in cases of statin intolerance or when another lipid-lowering drug fails [10].
Clinical practice guidelines are designed to assist physicians in optimizing patient treatment while minimizing the risk of serious adverse effects. These guidelines adhere to the Hippocratic oath's principle of "primum non nocere," meaning "first, do no harm." However, there is a concern for patients whose lipid profiles range from 97 to 135 mg/dL (2.5 to 3.49 mmol/L), as they may not qualify for initiation of PCSK9 therapy. Current guidelines recommend starting PCSK9 inhibitors when a patient's LDL-C reaches 135 mg/dL (3.5 mmol/L). This delay in management can result in atherosclerotic CVD which can inadvertently harm patients who, despite optimized lipid therapy, do not meet the established lipid targets [12].
Regrettably, patients may not meet the criteria for a PCSK9 inhibitor, a drug shown to reduce LDL-C levels by 50% in trials conducted by Raal et al. [13]. For these excluded patients, the issue often stems from previous diagnoses or treatments of comorbidities, such as CVD (e.g., ischemic stroke, cardiac arrest, peripheral vascular disease, or diabetic organ damage). The requirement for an LDL-C threshold exceeding 97 mg/dL (2.5 mmol/L) renders them ineligible for inclisiran, another PCSK9 inhibitor, which would otherwise be a suitable treatment option for patients with CVD. Healthcare providers, however, can still manage these patients by combining tolerable medications with lifestyle modifications.
The implementation of the NICE guidelines brings about substantial benefits. The established threshold of >135 mg/dL (>3.5 mmol/L) effectively helps triage patients, determining who requires PCSK9 inhibitors and who does not. This threshold simplifies the categorization of patients into high or very high-risk groups, regardless of their CVD history, facilitating a swift triage process by general practitioners. Additionally, it aids in prioritizing patients for further risk assessment using QRISK3 (ClinRisk Ltd). These guidelines have also streamlined the process for promptly referring patients who need urgent specialist consultation and have undergone therapy optimization but have not achieved LDL-C targets necessary for reducing overall cardiovascular risk. Another notable benefit of this threshold approach is its cost-effectiveness. Considering the higher costs associated with PCSK9 inhibitors relative to other treatments, it is prudent to reserve their use for those most likely to benefit significantly, rather than broadly prescribing them to all patients who have not met their treatment objectives [13].
The purpose of these guidelines is to establish a systematic method for using PCSK9 inhibitors, ensuring cost-effectiveness and proper resource allocation. It is important to recognize that the European Society of Cardiology (ESC)/European Atherosclerosis Society (EAS) criteria [14] have their drawbacks. While these criteria have proven effective for triaging patients who meet the threshold for initiating PCSK9 inhibitors, thereby saving costs and time, they also present challenges. Some individuals may not meet the thresholds of >135 mg/dL (>3.5 mmol/L) or >97 mg/dL (>2.5 mmol/L) required for starting alirocumab/evolocumab and inclisiran, respectively. This increases the cardiovascular risk for those who are unable to tolerate statins or other lipid-lowering drugs and do not meet the regulated threshold for PCSK9 inhibitor initiation. The LDL-C threshold set by these stringent ESC/EAS criteria may inadvertently exclude certain at-risk individuals, which seems counterintuitive. Additionally, some patients, even at their maximum tolerated dose, fall just short of the threshold by as little as 0.1 mmol/L, rendering them ineligible for PCSK9 inhibitor therapy. This situation presents a dilemma for specialists, leaving them with no further treatment options. A recent study by Bolodeoku et al. [15] revealed that out of 87 patients, 25 did not meet the threshold for using evolocumab (Repatha) and alirocumab (Praluent); however, these patients did meet the threshold criteria for inclisiran (Leqvio, Novartis) but lacked the comorbidities necessary for its consideration.
There is an urgent need to re-evaluate the LDL-C targets for initiating PCSK9 inhibitor therapy and for monitoring LDL-C levels. The rapid expansion of genomic data, coupled with the lack of appropriate clinical classifications for genetic variations, poses a significant challenge to traditional treatments for both common and genetic diseases. We should embark on a collaborative effort to thoroughly evaluate the functional and clinical implications of genomic variants. This will help bridge the gap and further the goals of personalized medicine. Such an effort includes conducting extensive global genomic sequencing of "healthy" individuals from various ethnic backgrounds [16]. Over the past decade, numerous pharmacogenomics and genome-wide association studies have identified many genetic variations that can influence drug efficacy (anti-lipid pharmacodynamics), absorption, metabolism, excretion (anti-lipid pharmacokinetics), and the mechanisms leading to anti-lipid toxicity [17]. These variations can make it challenging for some patient groups to achieve their targets, even when adhering to their medication regimen at the maximum tolerated dose. Addressing this issue may require dual therapy using different classes of lipid-lowering drugs. Several studies have suggested that intensive lipid-lowering therapy should aim for an LDL-C level of <1.4 mmol/L. For instance, a study by Nguyen et al. [18], revealed that 654 patients (89.2%) with high-risk CVD, particularly older individuals with type 2 diabetes (over 60 years), did not achieve the LDL-C goal of >54 mg/dL (<1.4 mmol/L). Being overweight was also identified as a factor contributing to the failure to meet LDL-C targets. Another study by Kim et al. [19] in Korea followed a cohort of 69,942 patients and reported a higher incidence of atherosclerotic CVD events among those who did not meet the LDL-C goal compared to those who did. Those who did not achieve the LDL-C goal were characterized as older individuals with higher cardiovascular risk levels than those who met the target. It is time to begin re-evaluating the criteria and LDL-C thresholds for prescribing PCSK9 inhibitors. The study by Bolodeoku et al. [15], as previously mentioned, demonstrated that LDL-C levels decreased significantly, ranging from 10% to 134%, with an average reduction of 60%. This variability in patient responses to PCSK9 inhibitors highlights the impact of biological differences and ongoing pharmacogenomic factors.
Over the past decade, numerous clinical trials, including ODYSSEY HIGH FH, FH I and II, LONG-TERM, COMBO I and II, OPTIONS I and II, MONO, and ALTERNATIVE, have been conducted on alirocumab, yielding a wealth of outcome data. Additionally, outcome data have been collected from secondary and tertiary healthcare centers running lipid clinics. The need for change becomes apparent as patients fail to achieve the expected LDL target levels, exposing them to an increased risk of CVD and escalating the financial burden on healthcare providers. Numerous outcome studies underscore this urgency, highlighting the need for collaborative efforts from ESC, EAS, and NICE to reassess individuals who do not meet the required targets. This is particularly critical for patients who may not meet the initiation threshold for inclisiran >97 mg/dL (>2.5 mmol/L) plus comorbidities such as stroke or CVD. Recent data on the use of inclisiran for lowering LDL-C are available, but there is no established evidence yet of its effectiveness in preventing cardiovascular risk [20]. Current clinical trials are ongoing to elucidate its effects and long-term outcomes on cardiovascular risk reduction. Furthermore, studies on its use in diverse populations are yet to be conducted, which is crucial for understanding its overall impact on reducing cardiovascular risk.
While acknowledging the constraints of time and financial considerations in these studies, there remains a strong argument for offering an alternative to patients who do not achieve prescribed targets despite being on maximum tolerated doses. Evidence from outcome studies suggests that PCSK9 inhibitors should be considered, given their demonstrated ability to reduce LDL levels by 50% to 60%. Another challenge is patient refusal to use injectable treatments. What are the options if a patient is intolerant to all medications in the lipid treatment pathways? Nevertheless, there is still potential for meeting treatment targets with these biologic therapies.
The ability to achieve LDL targets in patients who are intolerant to statins and are subsequently treated with PCSK9i is influenced by the presence or absence of CVD. Moreover, stricter controls are crucial for patients with cardiovascular risk to reduce overall mortality. According to the ESC, these controls are set at and 70 mg/dL (<1.8 mmol/L) and 70 mg/dL (<1.8 mmol/L). An analysis by Suresh et al. [21] showed that PCSK9 inhibitor monotherapy enabled 21.1% of patients to achieve an LDL-C goal of and 70 mg/dL (<1.8 mmol/L) and 5.3% to 70 mg/dL (<1.8 mmol/L), aligning with the NICE guidelines and the 2019 ESC/EAS guidelines, respectively. However, it was clear that fewer than 50% of patients were meeting these targets. Recently, NICE revised its guidelines to set the LDL-C target at 77 mg/dL (2.0 mmol/L), a move that represents a positive step towards achieving more realistic targets in real-world settings. This adjustment is expected to help more patients meet their LDL-C goals and reduce their overall cardiovascular risk.
PCSK9 inhibitors have revolutionized lipid management, especially for specialists treating patients who do not achieve their LDL targets and are at risk of cardiovascular events. However, it is essential to adhere to NICE guidelines for dyslipidaemia treatment to optimize patient care and minimize cardiovascular risk. These guidelines, with their specific LDL-C targets, may be unachievable for some due to biological variations or differences in drug metabolism pharmacogenomics. This issue becomes even more significant when starting treatment with a PCSK9 inhibitor. Therefore, experts should collaboratively reconsider and reassess these targets to ensure that no patient who could benefit from long-term lipid management is excluded.
The healthcare guidelines emphasize evidence-based decision-making, allowing lipidologists the flexibility to tailor and adjust treatment plans based on individual patient needs and comprehensive risk assessments. This approach includes evaluating cardiovascular risk and other associated comorbidities, which, if not addressed, could hinder achieving desired LDL targets. ESC and EAS must continue to adopt this perspective to optimize future treatments for patients on lipid-lowering therapy who do not meet expected LDL targets. Initiating an open discussion is essential for determining how to effectively manage these patients. Such a revision would provide an opportunity to refine and improve lipid management strategies, ensuring they better align with the varied needs and responses of individual patients. This revision also calls for a thoughtful and open discussion on revising the PCSK9 threshold.
Notes
REFERENCES
1. Bitton A, Gaziano TA. The Framingham Heart Study's impact on global risk assessment. Prog Cardiovasc Dis. 2010; 53:68–78.
2. Cullen P, Schulte H, Assmann G. The Münster Heart Study (PROCAM): total mortality in middle-aged men is increased at low total and LDL cholesterol concentrations in smokers but not in nonsmokers. Circulation. 1997; 96:2128–36.
3. Ference BA, Ginsberg HN, Graham I, Ray KK, Packard CJ, Bruckert E, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2017; 38:2459–72.
4. Steinberg D. Thematic review series: the pathogenesis of atherosclerosis. An interpretive history of the cholesterol controversy, part III: mechanistically defining the role of hyperlipidemia. J Lipid Res. 2005; 46:2037–51.
5. Steinberg D. Thematic review series: the pathogenesis of atherosclerosis. An interpretive history of the cholesterol controversy: part II. The early evidence linking hypercholesterolemia to coronary disease in humans. J Lipid Res. 2005; 46:179–90.
6. British Heart Foundation. UK factsheet: our vision is a world free from the fear of heart and circulatory diseases. British Heart Foundation; 2024.
7. Bradley CK, Wang TY, Li S, Robinson JG, Roger VL, Goldberg AC, et al. Patient-reported reasons for declining or discontinuing statin therapy: insights from the PALM registry. J Am Heart Assoc. 2019; 8:e011765.
8. Hirsh BJ, Smilowitz NR, Rosenson RS, Fuster V, Sperling LS. Utilization of and adherence to guideline-recommended lipid-lowering therapy after acute coronary syndrome: opportunities for improvement. J Am Coll Cardiol. 2015; 66:184–92.
9. Abifadel M, Varret M, Rabes JP, Allard D, Ouguerram K, Devillers M, et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet. 2003; 34:154–6.
10. Kim EJ, Wierzbicki AS. The history of proprotein convertase subtilisin kexin-9 inhibitors and their role in the treatment of cardiovascular disease. Ther Adv Chronic Dis. 2020; 11:2040622320924569.
11. National Institute for Health and Care Excellence (NICE). Alirocumab for treating primary hypercholesterolaemia and mixed dyslipidaemia: technology appraisal guidance. NICE; 2016.
12. Lin JL, Huang PH, Yeh HI, Li YH. Appropriate use of proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors for atherosclerotic cardiovascular disease: comparison of recommendations from different guidelines or consensus around the world. Acta Cardiol Sin. 2020; 36:403–8.
13. Raal FJ, Kallend D, Ray KK, Turner T, Koenig W, Wright RS, et al. Inclisiran for the treatment of heterozygous familial hypercholesterolemia. N Engl J Med. 2020; 382:1520–30.
14. Catapano AL, Graham I, De Backer G, Wiklund O, Chapman MJ, Drexel H, et al. 2016 ESC/EAS guidelines for the management of dyslipidaemias. Eur Heart J. 2016; 37:2999–3058.
15. Bolodeoku J, Gbaa T, Morris K, Whitehead S. Medicine optimisation using PCSK9 monoclonal antibodies (alirocumab and evolocumab) concerning the European Atherosclerosis Society (EAS) of < 1.8 mmol/l LDL cholesterol target in a secondary care lipid clinic. Br J Healthc Med Res. 2023; 10:91–102.
16. Carr DF, Francis B, Jorgensen AL, Zhang E, Chinoy H, Heckbert SR, et al. Genomewide association study of statin-induced myopathy in patients recruited using the UK clinical practice research datalink. Clin Pharmacol Ther. 2019; 106:1353–61.
17. Hayat M, Kerr R, Bentley AR, Rotimi CN, Raal FJ, Ramsay M. Correction: genetic associations between serum low LDL-cholesterol levels and variants in LDLR, APOB, PCSK9 and LDLRAP1 in African populations. PLoS One. 2021; 16:e0249478.
18. Nguyen HT, Ha KP, Nguyen AH, Nguyen TT, Lam HM. Non-achievement of the low-density lipoprotein cholesterol goal in older patients with type 2 diabetes mellitus and a very high cardiovascular disease risk: a multicenter study in Vietnam. Ann Geriatr Med Res. 2021; 25:278–85.
19. Kim S, Han S, Rane PP, Qian Y, Zhao Z, Suh HS. Achievement of the low-density lipoprotein cholesterol goal among patients with dyslipidemia in South Korea. PLoS One. 2020; 15:e0228472.
20. Ray KK, Raal FJ, Kallend DG, Jaros MJ, Koenig W, Leiter LA, et al. Inclisiran and cardiovascular events: a patient-level analysis of phase III trials. Eur Heart J. 2023; 44:129–38.
21. Suresh A, Theodoraki A, Ward E, Feher M. PCSK9 inhibitors and treatment targets: an audit-based evaluation of a specialist lipid clinic. Br J Diabetes. 2022; 22:30–35.
Fig. 1.
The chronological history of research on proprotein convertase subtilisin/kexin type 9 (PCSK9) and its applications, from basic science to clinical practice. The figure depicts the progression of research on PCSK9 and its applications, starting from basic science target validation, medication discovery, and clinical practice. Each milestone is separated by a 5-year interval. Phase 1–2 trials, light grey; phase 3 trials, medium grey; cardiovascular disease (CVD) outcome studies, dark grey; clinical guidelines in black boxes. Ab, antibody; ACS, acute coronary syndrome; ACC, American College of Cardiology; AHA, American Heart Association; ASO, antisense oligonucleotide; EAS, European Atherosclerosis Society; ESC, European Society of Cardiology; FH, familial hypercholesterolaemia; HoFH, homozygous FH; LDL-C, low-density lipoprotein cholesterol; NICE, National Institute for Health and Clinical Excellence; NLA, National Lipid Association USA; siRNA, small interfering RNA; SNP, single-nucleotide polymorphism; RNAi, RNA interference; TA, technological appraisal. Adapted from Kim et al. [10], available under the Creative Commons License.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download