Abstract
Background and Objectives
Natural killer T-cell lymphoma (NKTCL) and diffuse large B-cell lymphoma (DLBCL) are the two most prevalent subtypes of lymphoma in the sinonasal region. Accurately differentiating between sinonasal DLBCL and NKTCL is crucial for determining the appropriate treatment and prognosis. The present study compared the clinical characteristics of these two conditions.
Methods
We conducted a retrospective review of 173 patients diagnosed with sinonasal lymphoma at a single institute between 2004 and 2017. This review included only patients with DLBCL and NKTCL who had more than 6 months of follow-up records. We analyzed patient data encompassing clinical characteristics, pathologic findings, radiologic findings, treatment modalities, recurrence, and survival.
Results
Among the patients analyzed, 117 patients were diagnosed with NKTCL and 45 with DLBCL. Endoscopic evaluation revealed a significantly higher incidence of crusting (p<0.001) and necrotic lesions (p=0.001) in patients with NKTCL, whereas polypoid masses were more commonly observed in patients with DLBCL (p<0.001). Computed tomography (CT) scans indicated no significant differences in bilaterality or bone destruction between the two groups. The DLBCL group exhibited a higher rate of concurrent lymph node or organ involvement than the NKTCL group (p<0.001). The 5-year overall survival rate was 67.4% for DLBCL and 69.1% for NKTCL, with no significant difference between the two.
Non-Hodgkin lymphoma (NHL) is the second most common malignant tumor affecting the head and neck region [1]. However, extranodal sinonasal involvement is rare. Natural killer T-cell lymphoma (NKTCL) and diffuse large B-cell lymphoma (DLBCL) are the most common subtypes of extranodal NHL associated with the sinonasal tract [2,3].
DLBCL is a type of lymphoma that impacts B cells. It predominantly occurs in Western populations and typically affects males in their seventies. In contrast, NKTCL targets T lymphocytes and is more prevalent among Asian and Latin American males in their fifties. It is almost invariably linked to Epstein-Barr virus (EBV) infection [4-7].
DLBCL and NKTCL have different histologies, and it is important to distinguish between them for two main reasons. First, the treatment approaches for each subtype vary. In the case of DLBCL, treatment typically involves a chemotherapy regimen consisting of rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone (R-CHOP). In contrast, the treatment for NKTCL is tailored based on the patient’s overall health and the extent of the disease, with options ranging from localized to disseminated treatments. Recent guidelines suggest that a combination of chemoradiotherapy is more effective than chemotherapy alone for NKTCL patients when feasible, and non-anthracycline-based chemotherapy is preferred [8,9]. Second, NKTCL generally has a poorer prognosis compared to DLBCL, though this can vary with disease progression. The distinct histologies of these subtypes not only define their clinical characteristics but also contribute to the aggressive nature of NKTCL, which is known to cause necrosis of bone, cartilage, and soft tissue.
Despite its importance, the distinction between the two types of sinonasal lymphoma is not yet well understood. This study aimed to compare the clinical presentation, demographic data, and endoscopic and radiologic findings between DLBCL and NKTCL. The goal was to aid clinicians in making prompt and accurate diagnoses, preventing misdiagnosis, and effectively managing patients in clinical practice.
We retrospectively reviewed the medical records of patients diagnosed with sinonasal lymphoma at Asan Medical Center, a tertiary medical institute in South Korea, from July 2004 to September 2017. This study received approval from the Asan Medical Center Institutional Review Board (2022-0272). Pathological confirmation of the diagnoses was obtained for all patients, either at our institute or at other clinics.
During the specified period, 173 patients were treated for sinonasal lymphoma at our institute. Our study specifically focused on patients diagnosed with DLBCL (45 patients, 26.0%) and NKTCL (117 patients, 67.6%), totaling 162 patients. We excluded 14 patients from the study due to a follow-up period of less than 6 months, and an additional 8 patients who did not visit an otolaryngologist at our institute during their follow-up. Consequently, the study included 140 patients.
The staging of diseases was based on the Lugano classification, which evolved from the Ann Arbor staging system with the Cotswolds modifications [10]. This classification system categorizes patients into four stages, depending on the extent of the area involved [11]. Stages I and II are considered limited-stage, while stages III and IV are categorized as advancedstage.
To assess the rhinologic characteristics of the disease, the pre-treatment endoscopic records of 109 patients were examined by two rhinology specialists. The clinical features were evaluated using endoscopy photographs and the accompanying medical records. Where possible, rhinologic and orbital symptoms were also reviewed based on the medical records. Additionally, patient data including clinical characteristics, pathologic findings, radiologic findings, treatment modalities, recurrence, and survival rates were analyzed.
Tumor responses and recurrence were assessed by oncologists and rhinologists using nasal endoscopy, computed tomography (CT), magnetic resonance imaging (MRI), and 18F-FDG positron emission tomography/CT scans. The imaging was reviewed to evaluate bilaterality, bone destruction, involved lesions, orbital involvement, lymph node involvement, and systemic organ involvement.
The differences in endoscopic findings, rhinologic symptoms, and CT findings between DLBCL and NKTCL patients were reviewed and analyzed. Overall survival (OS), defined as the time from the first diagnosis to the patient’s death, and recurrence-free survival (RFS), defined as the time from the first diagnosis to the death or recurrence, were also calculated for each disease.
The data were analyzed using the Fisher exact test and the Student t-test. Five-year OS and RFS were calculated using the Kaplan–Meier method, and prognostic factors were analyzed using Cox proportional hazard analysis. Statistical analyses were conducted using SPSS Statistics for Windows, version 22.0 (IBM Corp., Armonk, NY, USA). Statistical significance was defined as p<0.05.
Among the 173 patients diagnosed with sinonasal lymphoma, the most common pathological type was NKTCL, observed in 117 patients (67.6%), followed by DLBCL in 45 patients (26.0%). Other pathological types included five cases of marginal zone lymphoma, three cases of peripheral T-cell lymphoma, one case of Burkitt lymphoma, and one case of mantle cell lymphoma. Additionally, one patient was diagnosed with an unspecified type of lymphoma. We conducted a study on 140 patients with either DLBCL or NKTCL to examine their clinical characteristics (Table 1). The average follow-up period was 68.3 months (range, 1.5–179.8 months).
The mean age of patients with DLBCL was higher than that of those with NKTCL (68.6 vs. 58.5 years), and the proportion of male patients was lower in the DLBCL group compared to the NKTCL group (58.1% vs. 66.0%). The prevalence of underlying conditions such as hypertension (34.9% vs. 16.5%) and diabetes (23.3% vs. 11.1%) was significantly higher in the DLBCL group than in the NKTCL group. EBV positivity in pathology was strongly associated with NKTCL, with 91 patients (93.8%) testing positive, whereas there were no EBV-positive patients in the DLBCL group. The proportion of patients with an advanced Ann Arbor stage was significantly higher in the DLBCL group compared to the NKTCL group (48.8% vs. 23.7%). The sex ratio, smoking, and alcohol consumption did not differ significantly between the two groups.
Out of 104 medical records analyzed, 25 cases of DLBCL and 79 cases of NKTCL were evaluated for rhinologic symptoms (Table 2). Nasal obstruction, purulent rhinorrhea, and crusting occurred significantly more often in NKTCL patients than in DLBCL patients. While epistaxis and foul-smelling discharge were also more common in NKTCL patients, these differences were not statistically significant. Conversely, orbital symptoms were significantly more prevalent in DLBCL patients compared to those with NKTCL. Orbital symptoms included orbital pain, decreased visual acuity and visual field, exophthalmos, ptosis, and binocular diplopia. Additionally, a decreased sense of smell was more frequent among DLBCL patients than NKTCL patients, though this difference was not statistically significant.
Endoscopic evaluations were documented in 109 cases, including 28 cases of DLBCL and 81 cases of NKTCL (Table 3). Endoscopic detection of the mass was successful in 17 (60.7%) of the DLBCL cases and 46 (56.8%) of the NKTCL cases. Necrotic lesions and crusting in the nasal cavity were observed more frequently in NKTCL than in DLBCL, while polypoid masses were more common in DLBCL than in NKTCL (Fig. 1). Septal perforation, fistulous tracts, and involvement of the hard palate were observed only in NKTCL patients; however, these differences were not statistically significant. Of the three fistula cases, two were naso-palatal and one was naso-cutaneous. There was no significant difference in the occurrence of purulent discharge and mucosal ulcers between the two groups. Disease progression was not related to endoscopic features in either group.
The radiologic findings for the DLBCL and NKTCL groups are presented in Table 4. In both groups, the nasal cavity was the most commonly affected site, followed by the maxillary sinus. There were no significant differences between the two groups in terms of bilaterality or the presence of destructive bone lesions. However, involvement of the orbit, lymph nodes, and extranodal organs was more frequent in the DLBCL group than in the NKTCL group.
The 5-year OS rate of DLBCL was 67.4% and that of NKTCL was 69.1%. There was no significant difference between the two groups. The 5-year RFS rate of DLBCL was 62.8% and that of NKTCL was 55.7%, again with no significant difference (Fig. 2).
We also evaluated the relationship between the clinical features of sinonasal lymphoma and prognosis through Cox regression analysis. This evaluation included all clinical features, such as patient characteristics, rhinologic and orbital symptoms, and findings from endoscopic and CT examinations, which were treated as independent variables in univariate analysis. However, we excluded the treatment modality from our analysis as it is not considered a prognostic factor. RFS was not associated with any of the clinical features we examined, but advanced disease stage was significantly linked to decreased OS (hazard ratio [HR], 2.66; 95% CI, 1.55–4.57; p< 0.001). Conversely, the presence of epistaxis was associated with increased OS (HR, 0.23; 95% CI, 0.05–0.94; p=0.042). We also explored the differences in OS and RFS between the two diseases while controlling for clinical features and significant prognostic factors identified in the univariate analysis, using multivariate analysis. In this analysis, the stage of the disease (HR, 2.41; 95% CI, 1.13–5.11; p=0.022) and epistaxis (HR, 0.22; 95% CI, 0.05–0.94; p=0.041) remained significant. However, the diagnosis of the patient was not significantly related to the prognosis (p=0.14). Other variables were also not significantly associated with differences in survival between the two diseases in the multivariate analysis.
In this study, we analyzed the differences between DLBCL and NKTCL by examining patient symptoms, endoscopic findings, and radiologic data. Our results revealed significant differences in rhinologic clinical manifestations between the two groups. This finding is clinically important because the two diseases require completely different treatment approaches. Understanding these distinct manifestations can aid clinicians in making a preliminary diagnosis before pathological confirmation is available [12,13]. To our knowledge, this is the first study to review the clinical rhinologic characteristics of sinonasal DLBCL and NKTCL.
Our analysis of epidemiology and disease characteristics aligns with previous studies conducted in other population groups. It is well established that DLBCL is prevalent in Caucasian populations, whereas NKTCL is more common in Asia and South America [6,14]. In our study, which focused on an Asian population, the majority of sinonasal lymphoma cases were identified as NKTCL. Other subtypes of sinonasal lymphoma, aside from DLBCL and NKTCL, represented only a small fraction of the cases.
The mean age of the NKTCL group was significantly lower than that of the DLBCL group, as were the numbers of patients with hypertension and diabetes. Previous literature has shown that DLBCL commonly occurs in male patients in their seventh decade, while NKTCL is more frequent in male patients in their fifth decade. This is consistent with our data [5-7].
NKTCL was highly associated with EBV: 91 of 97 NKTCL patients tested positive for EBV, whereas none of the DLBCL patients did. Various studies have analyzed the relationship between EBV infection and malignant lymphoma, noting that EBV has oncogenic properties that can affect lymphocytes and potentially influence the disease’s prognosis [6,15,16]. The distribution of disease stages differed significantly between the two groups in our study. The majority of NKTCL patients (76.3%) were diagnosed with limited-stage disease, while nearly half of the DLBCL patients (48.8%) presented with advanced-stage disease. Several reports from Asia have indicated that over 80% of sinonasal NKTCL cases are localized [17,18], whereas the proportion of patients with advanced disease among those with sinonasal DLBCL varies from 22.5% to 72% [19,20].
Regarding the sinonasal features of these diseases, Lombard et al. [20] reviewed 22 cases of sinonasal lymphoma, reporting symptoms such as nasal obstruction, mucopurulent rhinorrhea, and recurrent epistaxis. Additional symptoms included diplopia, exophthalmos, and facial swelling. We analyzed the rhinologic manifestations typically observed in each type of sinonasal lymphoma. Nasal obstruction was the most common symptom among both DLBCL and NKTCL patients. Notably, nasal obstruction, purulent rhinorrhea, and crusting were significantly more prevalent in NKTCL patients than in those with DLBCL. Furthermore, a higher percentage of NKTCL patients experienced epistaxis and foul-smelling discharge, although these differences were not statistically significant. Orbital symptoms and involvement were more frequently observed in the DLBCL group compared to the NKTCL group (Tables 2 and 4). Previous studies have also indicated that lymphomas of the B-cell lineage are more likely to present with orbital symptoms and extend into the orbit than lymphomas of the NK/T cell lineage [21].
In this study, we also examined the endoscopic findings reported by the rhinologist during the initial consultation. DLBCL was associated with significantly higher rates of polypoid masses compared to NKTCL. However, our data indicated that endoscopic examination was more likely to reveal necrosis and crusting in NKTCL than in DLBCL. The histopathological basis for this difference is that NKTCL typically exhibits an angiocentric and angio-destructive growth pattern, which leads to coagulative tissue necrosis [22]. Among patients with NKTCL, three had involvement of the hard palate and six had septal perforations; no such cases were observed in the DLBCL group. Although these findings were not statistically significant, we believe they can be attributed to the histopathologically destructive nature of NKTCL.
We analyzed the radiologic findings by examining the involved lesions, bilaterality, bone destruction, lymph node involvement, and the involvement of other organs. The most commonly affected site was the nasal cavity, followed by the maxillary sinus in both groups. Nasal cavity involvement was more prevalent in NKTCL than in DLBCL. Paranasal involvement was slightly more common in DLBCL than in NKTCL overall, with a significantly higher incidence of sphenoid sinus and orbital involvement in the DLBCL group. This finding aligns with a previous case series at Massachusetts General Hospital, which indicated that nasal involvement without sinus disease was more typical of NKTCL, while sinus involvement without nasal disease and orbital involvement were more frequently seen in DLBCL [21]. We concluded that the rates of bone destruction and bilateral manifestation did not differ significantly between the two diseases. However, the involvement of lymph nodes and other organs was higher in DLBCL than in NKTCL. This outcome is expected, as NKTCL is generally confined to the nasal cavity and septum, with most NKTCL patients presenting at a limited stage due to its local invasiveness [23]. Nevertheless, this does not imply that NKTCL is less aggressive than DLBCL, given that NKTCL typically exhibits extranodal and locally invasive characteristics, which are crucial prognostic factors.
In previous studies conducted in Western countries, sinonasal NKTCL was associated with a poorer prognosis compared to sinonasal DLBCL. Dubal et al. [24] conducted a retrospective analysis using data from the United States National Cancer Institute’s Surveillance Program, which showed that the 5-year disease-specific survival rate for sinonasal DLBCL was 63.5%, whereas it was only 30.9% for sinonasal NKTCL. However, the 5-year OS and RFS rates of DLBCL and NKTCL were not significantly different in our study. Recent studies targeting Asian populations have reported OS rates of 47%– 50%, which are similar to our results [25,26]. The standard management of NKTCL has evolved to include chemoradiation for localized disease and L-asparaginase-based systemic chemotherapy for advanced cases. Further research may be necessary to adequately compare the prognoses of these two diseases based on treatment modalities or racial differences.
We also evaluated the relationship between the clinical features of sinonasal lymphoma and prognosis using Cox regression analysis. Advanced-stage disease was associated with decreased OS, and epistaxis was related to increased OS. Both DLBCL and NKTCL patients with epistaxis tended to have limited disease—specifically, 16 NKTCL patients with epistaxis (84.2%) had limited disease, while 46 patients without epistaxis (76.7%) had limited disease, although the difference was not statistically significant. In the DLBCL group, all three patients with epistaxis had limited disease. A prominent symptom such as epistaxis might assist in the early diagnosis of the disease and lead to an improved prognosis.
This study had several limitations. First, there may have been selection bias in recruiting the study subjects due to the retrospective nature of the study and the relatively small size of the study population. However, given the rarity of sinonasal lymphoma, our study still provided significant insights into distinguishing between DLBCL and NKTCL, particularly regarding their clinical rhinologic manifestations. Second, the absence of MRI evaluation, which offers high diagnostic accuracy, also limited our investigation into the tumor entities themselves. Finally, although our analysis of endoscopic findings was conducted by rhinology specialists, there is currently no universally accepted method for describing these findings. We recommend the development of a globally standardized reporting system for endonasal lesions to facilitate easier diagnosis and enhance clinical research.
Our findings indicate that DLBCL and NKTCL exhibit distinct clinical presentations, endoscopic findings, and CT findings. DLBCL commonly involves lymph nodes and organs, typically presenting as a polypoidal mass. In contrast, NKTCL is more localized and is characterized by severe crusting and necrotic tissue upon endoscopic examination.
Notes
Availability of Data and Material
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Author Contributions
Conceptualization: Myeong Sang Yu. Data curation: Chol Ho Shin, Muna Aloraimi, Tae Gyeong Kim. Formal analysis: Chol Ho Shin, Muna Aloraimi. Investigation: Chol Ho Shin, Muna Aloraimi. Methodology: Chol Ho Shin, Muna Aloraimi. Supervision: Myeong Sang Yu. Writing—original draft: Chol Ho Shin, Muna Aloraimi. Writing—review & editing: Myeong Sang Yu.
References
1. Hermans R, Horvath M, De Schrijver T, Lemahieu SF, Baert AL. Extranodal non-Hodgkin lymphoma of the head and neck. J Belge Radiol. 1994; 77(2):72–7.
2. Kanumuri VV, Khan MN, Vazquez A, Govindaraj S, Baredes S, Eloy JA. Diffuse large B-cell lymphoma of the sinonasal tract: analysis of survival in 852 cases. Am J Otolaryngol. 2014; 35(2):154–8.

3. Vazquez A, Khan MN, Blake DM, Sanghvi S, Baredes S, Eloy JA. Extranodal natural killer/T-cell lymphoma: a population-based comparison of sinonasal and extranasal disease. Laryngoscope. 2014; 124(4):888–95.
4. Morton LM, Wang SS, Devesa SS, Hartge P, Weisenburger DD, Linet MS. Lymphoma incidence patterns by WHO subtype in the United States, 1992-2001. Blood. 2006; 107(1):265–76.

5. Smith A, Howell D, Patmore R, Jack A, Roman E. Incidence of haematological malignancy by sub-type: a report from the haematological malignancy research network. Br J Cancer. 2011; 105(11):1684–92.

6. Coha B, Vucinic I, Mahovne I, Vukovic-Arar Z. Extranodal lymphomas of head and neck with emphasis on NK/T-cell lymphoma, nasal type. J Craniomaxillofac Surg. 2014; 42(2):149–52.

7. Magrath I. The lymphoid neoplasms. 3rd ed. London: Hodder Arnold;2010.
8. van Doesum JA, Niezink AGH, Huls GA, Beijert M, Diepstra A, van Meerten T. Extranodal natural killer/T-cell lymphoma, nasal type: diagnosis and treatment. Hemasphere. 2021; 5(2):e523.
9. Chaganti S, Illidge T, Barrington S, Mckay P, Linton K, Cwynarski K, et al. Guidelines for the management of diffuse large B-cell lymphoma. Br J Haematol. 2016; 174(1):43–56.

10. Lister TA, Crowther D, Sutcliffe SB, Glatstein E, Canellos GP, Young RC, et al. Report of a committee convened to discuss the evaluation and staging of patients with Hodgkin’s disease: Cotswolds meeting. J Clin Oncol. 1989; 7(11):1630–6.

11. Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014; 32(27):3059–68.

12. Yamaguchi M, Suzuki R, Miyazaki K, Amaki J, Takizawa J, Sekiguchi N, et al. Improved prognosis of extranodal NK/T cell lymphoma, nasal type of nasal origin but not extranasal origin. Ann Hematol. 2019; 98(7):1647–55.

13. Chen Y, Wang X, Li L, Li W, Xian J. Differential diagnosis of sinonasal extranodal NK/T cell lymphoma and diffuse large B cell lymphoma on MRI. Neuroradiology. 2020; 62(9):1149–55.
14. Swain SK, Acharya S. Extranodal non-Hodgkin’s lymphoma of the sinonasal tract: a review. BLDE Univ J Health Sci. 2021; 6(1):1–6.
15. Ho FC, Srivastava G, Loke SL, Fu KH, Leung BP, Liang R, et al. Presence of Epstein-Barr virus DNA in nasal lymphomas of B and ‘T’ cell type. Hematol Oncol. 1990; 8(5):271–81.

16. Tao Q, Srivastava G, Loke SL, Liang RH, Liu YT, Ho FC. Epstein-Barr virus (EBV)-related lymphoproliferative disorder with subsequent EBV-negative T-cell lymphoma. Int J Cancer. 1994; 58(1):33–9.

17. Chim CS, Ma SY, Au WY, Choy C, Lie AK, Liang R, et al. Primary nasal natural killer cell lymphoma: long-term treatment outcome and relationship with the international prognostic index. Blood. 2004; 103(1):216–21.

18. Lee J, Suh C, Park YH, Ko YH, Bang SM, Lee JH, et al. Extranodal natural killer T-cell lymphoma, nasal-type: a prognostic model from a retrospective multicenter study. J Clin Oncol. 2006; 24(4):612–8.
19. Varelas AN, Eggerstedt M, Ganti A, Tajudeen BA. Epidemiologic, prognostic, and treatment factors in sinonasal diffuse large B -cell lymphoma. Laryngoscope. 2019; 129(6):1259–64.

20. Lombard M, Michel G, Rives P, Moreau A, Espitalier F, Malard O. Extranodal non-Hodgkin lymphoma of the sinonasal cavities: a 22-case report. Eur Ann Otorhinolaryngol Head Neck Dis. 2015; 132(5):271–4.

21. Cuadra-Garcia I, Proulx GM, Wu CL, Wang CC, Pilch BZ, Harris NL, et al. Sinonasal lymphoma: a clinicopathologic analysis of 58 cases from the Massachusetts General Hospital. Am J Surg Pathol. 1999; 23(11):1356–69.
22. Schmitt C, Sako N, Bagot M, Huang Y, Gaulard P, Bensussan A. Extranodal NK/T-cell lymphoma: toward the identification of clinical molecular targets. J Biomed Biotechnol. 2011; 2011:790871.

23. Yan Z, Huang HQ, Wang XX, Gao Y, Zhang YJ, Bai B, et al. A TNM staging system for nasal NK/T-cell lymphoma. PLoS One. 2015; 10(6):e0130984.
24. Dubal PM, Dutta R, Vazquez A, Patel TD, Baredes S, Eloy JA. A comparative population-based analysis of sinonasal diffuse large B-cell and extranodal NK/T-cell lymphomas. Laryngoscope. 2015; 125(5):1077–83.
Fig. 1.
Endoscopic findings of DLBCL and NKTCL patients. A: DLBCL tends to present with a polypoidal mass. B: NKTCL tends to present with crusting and necrotic tissue. DLBCL, diffuse large B-cell lymphoma; NKTCL, natural killer T-cell lymphoma.
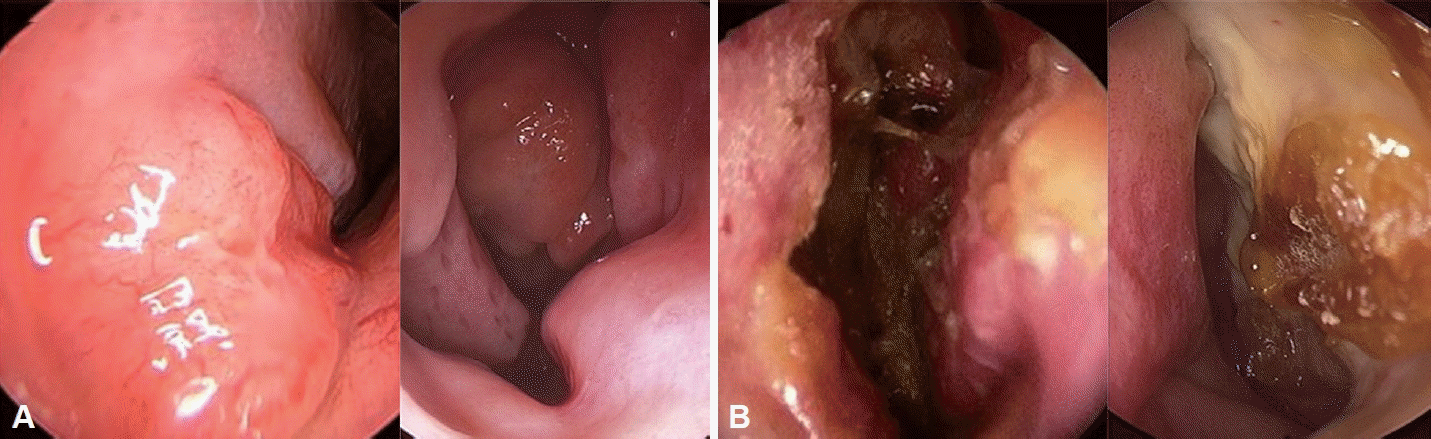
Fig. 2.
Five-year overall survival (OS) and recurrence-free survival (RFS) rates of DLBCL and NKTCL patients. A: The 5-year OS of DLBCL was 67.4%, and that of NKTCL was 69.1%. The difference was not statistically significant (p=0.647). B: The 5-year RFS of DLBCL was 62.8%, and that of NKTCL was 55.7%. The difference was not statistically significant (p=0.953). DLBCL, diffuse large B-cell lymphoma; NKTCL, natural killer T-cell lymphoma.
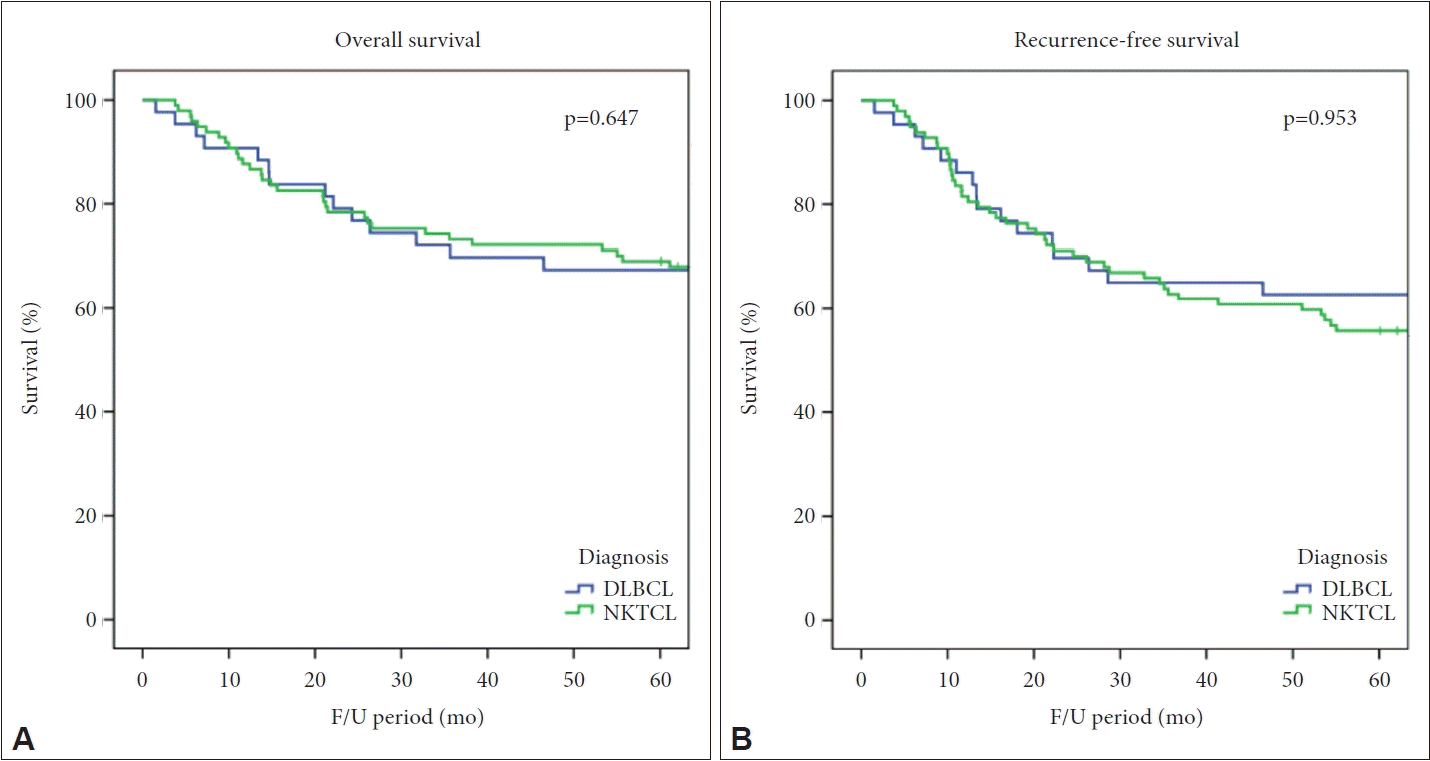
Table 1.
Comparison of characteristics between DLBCL and NKTCL patients (n=140)
| Variable | DLBCL (n=43) | OR (95% CI) | NKTCL (n=97) | OR (95% CI) | p* |
|---|---|---|---|---|---|
| Age (yr), mean±SD | 68.6±12.0 | - | 58.5±13.2 | - | <0.001 |
| Sex, male | 25 (58.1) | 0.71 (0.34–1.50) | 64 (66.0) | 1.40 (0.67–2.92) | 0.374 |
| HTN | 15 (34.9) | 2.71 (1.19–6.19) | 16 (16.5) | 0.37 (0.16–0.84) | 0.016 |
| DM | 10 (23.3) | 2.63 (1.00–6.91) | 10 (11.1) | 0.38 (0.15–0.99) | 0.043 |
| History of smoking (n=108) | 5/18 (27.8) | 0.52 (0.17–1.60) | 38/90 (42.2) | 1.90 (0.62–5.78) | 0.253 |
| Alcohol consumption (n=108) | 6/18 (33.3) | 0.79 (0.27–2.29) | 35/90 (38.9) | 1.27 (0.44–3.70) | 0.657 |
| EBV positivity (n=139) | 0/42 (0.0) | - | 91/97 (93.8) | - | <0.001 |
| Ann Arbor staging | 0.003 | ||||
| Limited | 22 (51.2) | 74 (76.3) | |||
| Advanced | 21 (48.8) | 3.07 (1.44–6.56) | 23 (23.7) | 0.33 (0.15–0.70) | |
| Primary treatment modality | <0.001 | ||||
| Chemotherapy only | 43 (100) | 36 (37.1) | |||
| CCRT | 0 | - | 61 (62.9) | - | |
| Recurrence, any site | 10 (23.8) | 0.69 (0.30–1.58) | 30 (31.3) | 1.46 (0.63–3.34) | 0.375 |
Table 2.
Comparison of rhinologic and orbital symptoms between DLBCL and NKTCL in sinonasal lymphoma patients (n=104)
| Variable | DLBCL (n=25) | OR (95% CI) | NKTCL (n=79) | OR (95% CI) | p* |
|---|---|---|---|---|---|
| Nasal obstruction | 13 (52.0) | 0.23 (0.09–0.62) | 65 (82.3) | 4.29 (1.62–11.4) | 0.002 |
| Purulent rhinorrhea | 7 (28.0) | 0.31 (0.12–0.82) | 44 (55.7) | 3.23 (1.21–8.61) | 0.016 |
| Epistaxis | 3 (12.0) | 0.43 (0.12–1.60) | 19 (24.1) | 2.32 (0.63–8.62) | 0.266 |
| Loss of odor | 6 (24.0) | 1.47 (0.50–4.33) | 14 (17.7) | 0.68 (0.23–2.02) | 0.488 |
| Foul-smelling | 0 | - | 10 (12.7) | - | 0.113 |
| Crusting | 5 (20.0) | 0.26 (0.09–0.75) | 39 (49.4) | 3.90 (1.33–11.4) | 0.010 |
| Orbital symptoms (n=135) | 7/43 (16.3) | 18.7 (2.22–157) | 1/92 (1.1) | 0.06 (0.01–0.48) | 0.001 |
Table 3.
Comparison of endoscopic and computed tomography findings between DLBCL and NKTCL in sinonasal lymphoma patients (n=109)
| Variable | DLBCL (n=28) | OR (95% CI) | NKTCL (n=81) | OR (95% CI) | p* |
|---|---|---|---|---|---|
| Presence of endonasal mass | 16 (57.1) | 1.01 (0.43–2.42) | 46 (56.8) | 0.99 (0.41–2.35) | 0.665 |
| Necrosis | 3 (10.7) | 0.14 (0.04–0.51) | 37 (45.7) | 7.01 (1.96–25.1) | 0.001 |
| Septal perforation | 0 | - | 6 (7.4) | - | 0.335 |
| Polypoid mass | 8 (28.6) | 10.3 (2.49–42.3) | 3 (3.8) | 0.10 (0.02–0.40) | <0.001 |
| Severe crusting | 4 (14.3) | 0.13 (0.04–0.42) | 45 (55.6) | 7.50 (2.39–23.6) | <0.001 |
| Purulent discharge | 11 (39.3) | 0.70 (0.29–1.67) | 39 (48.1) | 1.44 (0.60–3.44) | 0.417 |
| Mucosal ulcers | 5 (17.9) | 0.62 (0.21–1.84) | 21 (25.9) | 1.61 (0.54–4.78) | 0.388 |
| Fistulous tract (skin or oral cavity) | 0 | - | 3 (3.7) | - | 0.568 |
| Hard palate involvement | 0 | - | 3 (3.7) | - | 0.568 |
Table 4.
Computed tomography findings in sinonasal lymphoma patients with DLBCL or NKTCL (n=140)
| Variable | DLBCL (n=43) | OR (95% CI) | NKTCL (n=97) | OR (95% CI) | p* |
|---|---|---|---|---|---|
| Bilaterality | 10 (23.3) | 0.68 (0.30–1.55) | 30 (30.9) | 1.48 (0.65–3.38) | 0.354 |
| Bone destruction | 6 (14.0) | 0.76 (0.29–2.09) | 17 (17.5) | 1.31 (0.48–3.59) | 0.599 |
| Involved lesion | |||||
| Nasal cavity | 32 (74.4) | 0.06 (0.01–0.29) | 95 (97.9) | 16.3 (3.44–77.6) | <0.001 |
| Maxillary sinus | 22 (51.2) | 1.32 (0.64–2.70) | 43 (44.3) | 0.76 (0.37–1.56) | 0.455 |
| Ethmoid sinus | 17 (39.5) | 1.16 (0.55–2.42) | 35 (36.1) | 0.86 (0.41–1.81) | 0.697 |
| Frontal sinus | 4 (9.3) | 1.14 (0.32–4.01) | 8 (8.2) | 0.88 (0.25–3.08) | 0.534 |
| Sphenoid sinus | 9 (20.9) | 8.29 (2.12–32.5) | 3 (3.1) | 0.12 (0.03–0.47) | 0.001 |
| Orbit involvement | 9 (20.9) | 12.6 (2.59–61.1) | 2 (2.1) | 0.08 (0.02–0.39) | <0.001 |
| Lymph node involvement | 18 (41.9) | 6.26 (2.57–15.3) | 10 (10.3) | 0.16 (0.07–0.39) | <0.001 |
| Organ involvement outside sinonasal area | 17 (39.5) | 31.1 (6.74–143) | 2 (2.1) | 0.03 (0.01–0.15) | <0.001 |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download