Abstract
Tuberculosis of the paranasal sinus is a rare disease caused by infection with Mycobacterium tuberculosis, the causative agent of pulmonary tuberculosis. Although the worldwide incidence of tuberculosis is declining, the diagnosis of primary paranasal tuberculosis remains challenging and cannot be ruled out in patients presenting with refractory chronic rhinosinusitis after adequate surgical and medical treatment. We experienced a case of paranasal tuberculosis with no evidence of previous tuberculosis infection. It was diagnosed after a surgical biopsy revealed granulomatous inflammation and caseous necrosis. The patient responded well to antituberculosis drug therapy and became free of symptoms after 7 months of treatment. We report our findings in this case with a review of the recent literature.
Tuberculosis is a chronic infectious disease caused by Mycobacterium tuberculosis. It is known to cause disease in almost all tissues or organs of the human body, although most cases are pulmonary. In active pulmonary tuberculosis, patients may be directly infected by bacteria in the atmosphere or by bacteria in sputum or hemoptysis, which invade damaged mucous membranes in the nasal cavity or sinus, spreading to lymph nodes or the bloodstream. According to data from the Korea Tuberculosis Association, the total number of new tuberculosis patients in 2021 was 18,335 (35.7 per 100,000), a decrease of 8.0% (1,598) from 2020 (19,933 new patients; 38.8 per 100,000). It has decreased by more than half over the past decade, and the number of new extrapulmonary tuberculosis patients in 2021 also declined to 4,235 (8.3 per 100,000) over the past decade [1].
Extrapulmonary tuberculosis is known to occur secondary to pulmonary tuberculosis, but in rare cases, tuberculosis that invades the upper respiratory tract may occur primarily. The prevalence of primary tuberculosis of the nasal cavity and paranasal sinuses is extremely low. Moreover, it is difficult to diagnose because the clinical symptoms are nonspecific and must be distinguished from other necrotic and granulomatous diseases. Thus, a higher degree of clinical suspicion is important to facilitate rapid diagnosis and treatment.
We report a recent case of primary sinonasal tuberculosis and review the literature that describes this rare disease.
A 42-year-old woman presented with complaints of left hearing loss and tinnitus, which had persisted for 4 months. She also complained of rhinorrhea, especially on the left side, and nasal obstruction on both sides. Her general health was excellent and she had no other symptoms.
On physical examination, she had a non-erythematous and dull tympanic membrane with suspicious mucoid fluid on the left side, suggesting suppurative otitis media. In addition, nasal endoscopy showed purulent discharge in the left nasal cavity and fibrotic changes in the mucosa surrounding the left pharyngeal orifice of the eustachian tube. There were no palpable neck lymph nodes. A routine preoperative workup, including hematology results and a chest X-ray examination, was unremarkable. A skin prick test and blood test for allergies were negative.
Paranasal computed tomography (CT) revealed diffuse opacification of the left maxillary, ethmoid, and frontal sinuses, and soft tissue density in the left middle ear cavity (Fig. 1). There was no bony erosion or other specific findings on the CT scan images.
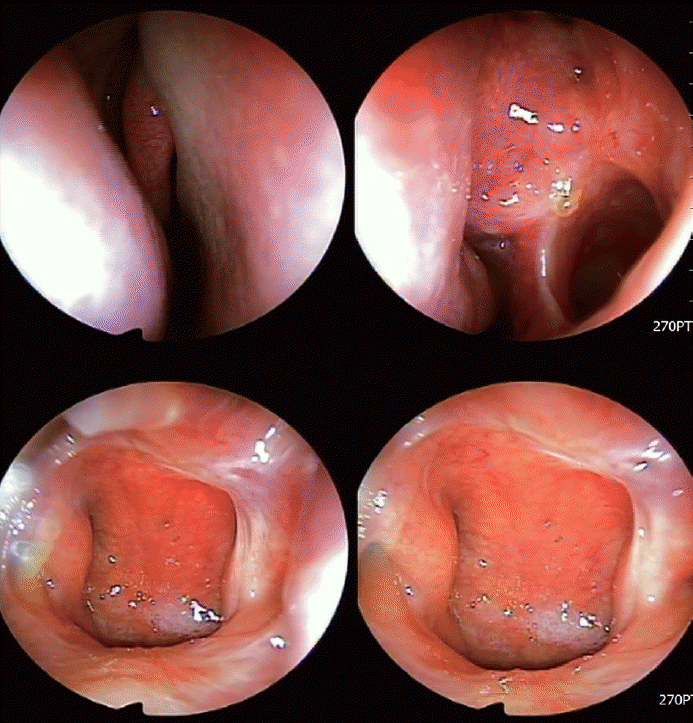
The patient underwent endoscopic sinus surgery (ESS). Mucopus was identified in the left middle meatus and inside the maxillary sinus during surgery. When we evaluated the whole nasal cavity and nasopharynx, an unusual necrotizing lesion was observed at the posterior aspect of the nasal septum and nasal floor of the left nasal cavity (Fig. 2). We harvested mucosal tissues from the necrotizing lesion on the posterior nasal septum and nasal floor and performed a biopsy, tuberculosis culture, Epstein-Barr virus (EBV) antibody test, and tuberculosis/nontuberculosis mycobacteria (TB/NTM) real-time polymerase chain reaction (RT-PCR) test. In addition, we found that the left maxillary sinus was covered by thickened mucosa, suggesting inflammatory changes. We also performed a biopsy of the left maxillary sinus. After ESS, a left ventilation tube was inserted due to suspicion of left suppurative otitis media.
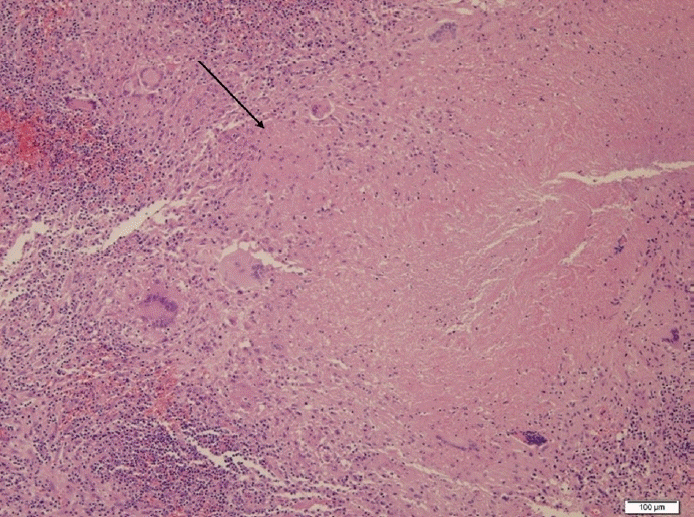
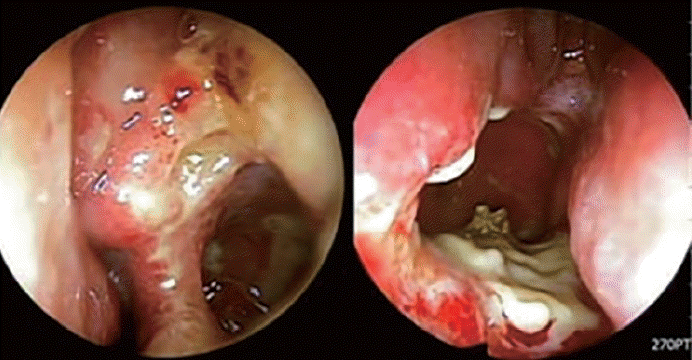
The patient was discharged 2 days after surgery and prescribed nasal saline irrigation, intranasal corticosteroid spray, mucolytics, and oral antibiotics. We received the final pathology report showing necrotic granuloma in the left maxillary sinus mucosa and left nasal septum posterior mucosa (Fig. 3). The EBV antibody test and TB/NTM RT-PCR test were negative. During the routine postoperative care, the patient complained of several symptoms, including left nasal obstruction, persistent left mucopurulent discharge, ear discharge, and newly occurring facial pain. Nasal endoscopic examination revealed persistent necrotic mucosa in the left nasal cavity (Fig. 4). We received the tuberculosis culture test results 1 month postoperatively showing M. tuberculosis complex. We referred the patient to the Department of Infectious Diseases. As the chest radiography performed before surgery showed normal findings, the patient was diagnosed with primary paranasal tuberculosis, and antituberculosis drug therapy was started. After 1 month of treatment, symptoms improved along with the disappearance of the necrotic lesion (Fig. 5). Antituberculosis medication was continued for 7 months, and the follow-up TB/NTM RT-PCR test and tuberculosis culture of the nasal mucosa were negative.
Primary tuberculosis infection of the nasal cavity and paranasal sinuses is a rare condition. The extrapulmonary form of tuberculosis occurs secondarily in only 15% of tuberculosis patients, and primary paranasal tuberculosis is extremely rare [2]. It was first reported in 1761 by Giovanni Morgagni, who studied the impact of tuberculosis on the human body [3]. In a review of English-language medical literature spanning 95 years, Butt [4] identified only 35 patients with primary nasal tuberculosis. In the upper respiratory tract, the nose and paranasal sinuses are the anatomical regions most resistant to tuberculous invasion because of their unique epithelial architecture and bactericidal secretions. However, this region can still be affected by inhalation of infected particles, an immunodeficiency status, traumatic inoculation, or a combination of these factors [5].
Primary paranasal tuberculosis is twice as common in women than in men, and the median age is 40 years [6]. It usually presents unilaterally but can be bilateral in one-third of patients. The lesion can be proliferative, infiltrative, or ulcerative. As with our patient, primary paranasal tuberculosis commonly affects the nasal septum first, followed by the paranasal sinuses, choana, nasopharynx, and anterior segment of the inferior turbinate [7]. Patients usually present with nasal obstruction, bloody nasal discharge, crusting, pain, dryness of the nose and throat, epiphora, or postnasal discharge [8-11]. In advanced cases, it can extend to orbital involvement.
The diagnosis of paranasal tuberculosis requires nasal examination with findings such as granulomatous nasal mucosa, ulcerative lesions, and bright red nodular hypertrophy, as well as chest X-ray, biopsy of intranasal lesions, and identification of tuberculosis bacteria in the patient’s secretions or tissue culture [12]. However, the diagnosis of primary paranasal tuberculosis is challenging and can be potentially misdiagnosed because it is rare and not commonly encountered in clinics. Moreover, it often mimics other relatively common diseases such as fungal rhinosinusitis, sinonasal neoplasms (including inverted papilloma and lymphoma), and other granulomatous diseases [9].
Several diseases that require differentiation from tuberculosis include granulomatosis with polyangiitis (GPA; formerly known as Wegener’s granulomatosis), extranodal NK/T-cell lymphoma (ENTKL), and sarcoidosis. GPA is an autoimmune disease characterized by necrotizing granulomas, vasculitis of the upper and lower respiratory tracts, and necrotizing glomerulonephritis [13]. Rhinological symptoms are the most common ear, nose, and throat (ENT) manifestations, occurring in 41% of cases and including nasal obstruction, discharge, and anosmia [14]. A previous study reported sinonasal involvement in 89% of patients, with 61% meeting the criteria for chronic rhinosinusitis (CRS) [15]. Other possible manifestations included septal perforation (33%) and saddle nose deformity (23%). Diagnosis is based on clinical history, nasal endoscopy findings, lab data (urinalysis, complete blood count, metabolic panel, erythrocyte sedimentation rate, hemoglobin, serum creatinine, antineutrophil cytoplasmic antibodies [ANCA] incluing cytoplasmic-ANCA [c-ANCA] and perinuclear-ANCA [p-ANCA]), and biopsy. While c-ANCA is highly sensitive for Wegener’s granulomatosis, a negative result does not exclude the disease. Histopathological examination typically reveals intramural, eccentric, necrotizing granulomatous lesions in small to medium vessels. CT imaging can show extensive destruction of the paranasal sinus with bony erosion and active osteoneogenesis. Sarcoidosis is a systemic granulomatous disease that can affect various organs, including the lungs, liver, kidneys, central nervous system, and head and neck structures like the salivary glands, larynx, sinuses, eyes, ears, and lymph nodes. The most common ENT symptom is nasal obstruction, with the nasal mucosa often showing a “strawberry skin” appearance due to granulomas [16]. Like GPA, septal perforation and saddle nose deformity may occur. Diagnosis is made by excluding other conditions such as tuberculosis, fungal infections, cancer, and vasculitis. Elevated serum angiotensin-converting enzyme levels are found in 80% of sarcoidosis cases. A biopsy of nasal nodules showing noncaseating granulomas can support the diagnosis of sarcoidosis [17]. Treatment typically involves systemic steroids, with surgical intervention reserved for cases with significant obstruction, mucocele formation, or extension to adjacent structures. ENTKL is a subtype of mature T- and NKcell lymphoma associated with EBV, predominantly affecting the nasal and paranasal regions [18]. Early signs and symptoms are nonspecific, but advanced disease may present with systemic symptoms such as night sweats, fever, joint pain, fatigue, and rapid destruction of the ipsilateral head and neck region. Diagnosis requires histological and immunofluorescence testing that shows polymorphic lymphoid infiltration, coagulative necrosis, and vascular destruction [19]. Immunofluorescence typically shows positivity for CD2 and CD56 and negativity for CD5, CD4, and CD8. ENT specialists play a crucial role in removing necrotic tissue and obtaining accurate biopsy samples for diagnosis.
The clinician’s careful and thorough examination with a high degree of suspicion is crucial for diagnosis. As shown in this case, the confirmative diagnosis for paranasal tuberculosis is based on the results of Ziehl–Neelsen staining for acidfast bacillus and the culture of organisms obtained from nasal tissue or secretions.
In this case, based on the subjective symptoms and objective image findings, the initial impression was unilateral CRS. However, we found unusual ulcerative lesions on the posterior nasal passage and nasopharynx, which were confirmed as M. tuberculosis complex. Since there were no abnormal findings on the preoperative chest X-ray, we concluded that the patient’s final diagnosis was primary paranasal tuberculosis. As shown in this case, paranasal tuberculosis can potentially be misdiagnosed as CRS or other inflammatory diseases. The administration of empirical antibiotics as the initial treatment was understandable and, by exclusion, may have indirectly contributed to the establishment of sinonasal tuberculosis as the diagnosis [6]. However, following biopsy in a patient with a high index of suspicion, initiating anti-tubercular treatment should be considered to save time until the diagnosis is confirmed and to avoid the unnecessary use of antibiotics [6].
For the treatment of primary paranasal tuberculosis, multidrug antituberculosis therapy for a minimum of 6 months is the mainstay of treatment, accompanied by sufficient removal of the involved nasal mucus and crust. In this case, the patient received 7 months of multidrug antituberculosis therapy, and we confirmed remission of the lesions and improvement of the subjective symptoms.
In conclusion, we recently experienced a case of primary paranasal tuberculosis, which was diagnosed and treated successively. Since primary paranasal tuberculosis is rare and commonly shows nonspecific clinical features, clinicians should maintain a high degree of suspicion that considers the possibility of this diagnosis so that patients receive early diagnosis and treatment.
Notes
Ethics Statement
This study was granted ethical approval by the Institutional Review Board of Kyung Hee University Hospital (KHUH 2022-07-071). The consents from the patient was waived because of excluding the information of the patient.
Availability of Data and Material
Data sharing not applicable to this article as no datasets were generated or analyzed during the study.
References
1. Kim J, Lee H, In H, Kim Y. [Characteristics of the notified tuberculosis: the Republic of Korea, 2021]. Public Health Wkly Rep. 2022; 15(12):739–46. Korean.
2. Lee MS, Kim DJ, Kim MS, Sung KJ, Park DJ. [Sinonasal tuberculosis: report of two cases demonstrated with CT]. J Korean Radiol Soc. 1999; 41(6):1107–9. Korean.
3. Sanehi S, Dravid C, Chaudhary N, Venkatachalam VP. Tuberculosis of paranasal sinuses. Indian J Otolaryngol Head Neck Surg. 2008; 60(1):85–7.

5. Dey S, Misra S, Dutta M. Primary sinonasal tuberculosis: a diagnostic challenge. Turk Arch Otorhinolaryngol. 2018; 56(2):117–21.

6. Saha K, Mitra M, Saha A, Barma P. Primary nasal tuberculosis following blunt trauma nose. Med J Dr D.Y. Patil Univ. 2014; 7(1):50–2.
7. Sethi A, Agarwal AK, Girhotra M, Naithani P. Tuberculosis: an extremely unusual cause of orbital wall erosion. Orbit. 2011; 30(2):101–4.
8. Alavi SM, Nashibi R. Nasal tuberculosis in a 56 year old woman. Caspian J Intern Med. 2014; 5(1):49–51.
9. Khan S, Pujani M, Jetley S. Primary nasal tuberculosis: resurgence or coincidence−a report of four cases with review of literature. J Lab Physicians. 2017; 9(1):26–30.
10. Jablenska L, Lo S, Uddin J, Toma A. Nasolacrimal tuberculosis: case report highlighting the need for imaging in identifying and managing it effectively. Orbit. 2010; 29(2):126–8.

11. Bhandare CA, Barad PS. Lupus vulgaris with endopthalmitis--a rare manifestation of extrapulmonary tuberculosis in India. Indian J Tuberc. 2010; 57(3):98–101.
12. Hwang JH, Jin DS, Chai YH, Lee KO. [Two cases of primary tuberculosis arising in nasal cavity]. Korean J Otorhinolaryngol-Head Neck Surg. 1995; 38(8):1273–6. Korean.
13. Jennette JC, Falk RJ, Andrassy K, Bacon PA, Churg J, Gross WL, et al. Nomenclature of systemic vasculitides. Arthritis Rheum. 1994; 37(2):187–92.

14. Srouji IA, Andrews P, Edwards C, Lund VJ. Patterns of presentation and diagnosis of patients with Wegener’s granulomatosis: ENT aspects. J Laryngol Otol. 2007; 121(7):653–8.

15. Cannady SB, Batra PS, Koening C, Lorenz RR, Citardi MJ, Langford C, et al. Sinonasal Wegener granulomatosis: a single-institution experience with 120 cases. Laryngoscope. 2009; 119(4):757–61.

16. Aloulah M, Manes RP, Ng YH, Fitzgerald JE, Glazer CS, Ryan MW, et al. Sinonasal manifestations of sarcoidosis: a single institution experience with 38 cases. Int Forum Allergy Rhinol. 2013; 3(7):567–72.

17. Wilson R, Lund V, Sweatman M, Mackay IS, Mitchell DN. Upper respiratory tract involvement in sarcoidosis and its management. Eur Respir J. 1988; 1(3):269–72.
18. Haverkos BM, Pan Z, Gru AA, Freud AG, Rabinovitch R, Xu-Welliver M, et al. Extranodal NK/T cell lymphoma, nasal type (ENKTL-NT): an update on epidemiology, clinical presentation, and natural history in North American and European cases. Curr Hematol Malig Rep. 2016; 11(6):514–27.

19. Trimarchi M, Gregorini G, Facchetti F, Morassi ML, Manfredini C, Maroldi R, et al. Cocaine-induced midline destructive lesions: clinical, radiographic, histopathologic, and serologic features and their differentiation from Wegener granulomatosis. Medicine (Baltimore). 2001; 80(6):391–404.
Fig. 1.
Preoperative computed tomography (CT) images. Coronal view (A) and axial view (B) of CT of the paranasal sinus showing heterogenous soft tissue density in the left maxillary, anterior ethmoid, and frontal sinuses with the left middle ear cavity.
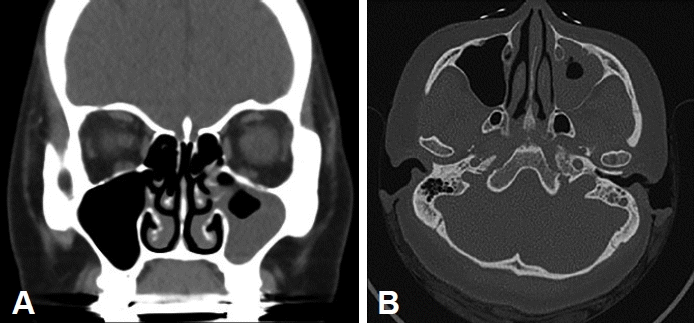
Fig. 2.
Intraoperative endoscopic findings of the nasal cavity and nasopharynx, showing granulomatous inflammation with necrosis.
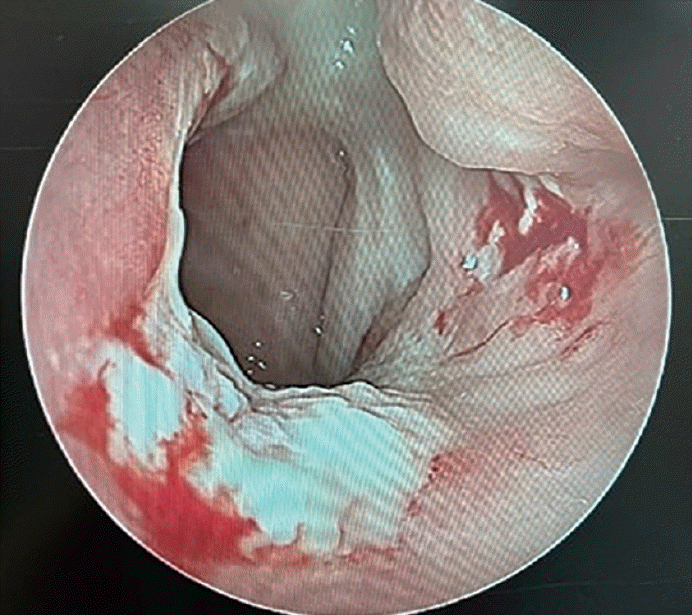
Fig. 3.
Hematoxylin and eosin-stained section of the nasal mucosa (×100). Infiltrations with lymphocytes, macrophages, and possible multinucleated giant cells, and caseating necrosis (a form of cell death where the tissue turns into a soft, white, and cheese-like material) were shown (marked by arrow line).




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download