Some of the included studies delineated changes in individual TNSS subdomain scores; thus, changes in these subdomains were also scrutinized in our review. All subdomains changed significantly from the baseline during all follow-up periods (
Fig. 3): congestion scores at 1 month (mean difference [95% CI]: 1.0372 [0.8890–1.1855]; I
2=38.9%), 3 months (1.0680 [0.9123–1.2237], I
2=43.8%), 6 months (1.2192 [0.9734– 1.4651]; I
2=78.5%), and 12 months (1.3865 [1.2644–1.5086]; I
2=0.0%) (p=0.0009); itching scores at 1 month (0.4660 [0.3356– 0.5965]; I
2=54.7%), 3 months (0.4320 [0.2963–0.5678]; I
2= 60.7%), 6 months (0.4361 [0.2744–0.5979]; I
2=81.0%), and 12 months (0.5000 [0.2171–0.7829]; I
2=NA) (p=0.9649); rhinorrhea scores at 1 month (1.1634 [0.8904–1.4364]; I
2=76.1%), 3 months (1.2632 [1.1338–1.3927]; I
2=0.0%), 6 months (1.3000 [1.1589–1.4411]; I
2=0.0%), and 12 months (0.9560 [0.4204– 1.4915]; I
2=71.3%); and sneezing scores at 1 month (0.4563 [0.3218–0.5908]; I
2=62.5%), 3 months (0.6701 [0.5276–0.8127]; I
2=40.3%), 6 months (0.5881 [0.4271–0.7491]; I
2=31.6%), and 12 months (0.6400 [0.2711–1.0089]; I
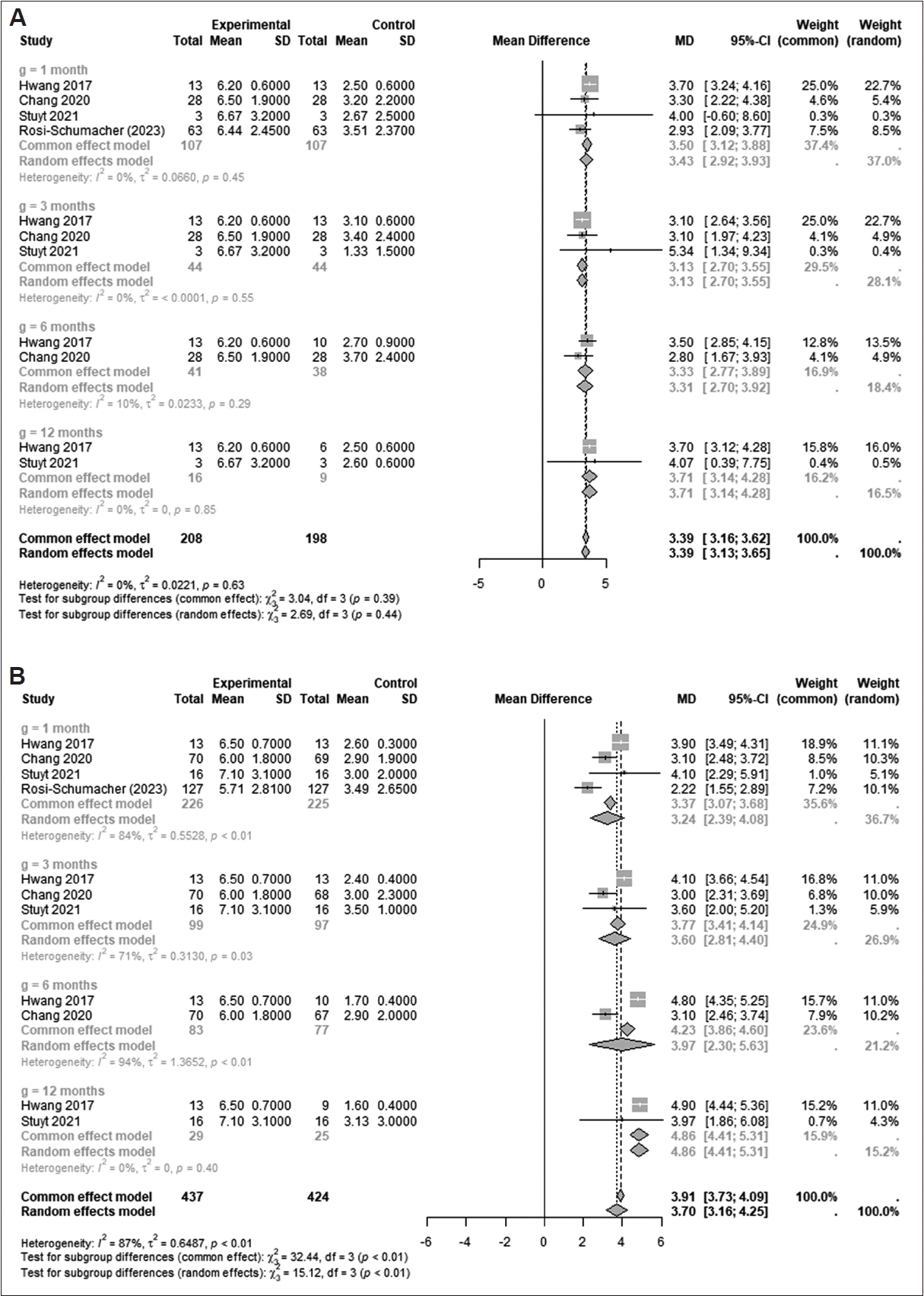
2=NA) (p=0.1850). In particular, the mean difference of congestion scores from baseline increased significantly over time (from 1 month to 12 months after treatment; p=0.0009). These results showed that cryoablation would be beneficial for all subdomains of nasal symptoms, especially congestion, in chronic rhinitis. Because some included studies measured changes in outcomes by rhinitis type (allergic rhinitis [AR] or NAR), we also evaluated changes based on the type of rhinitis. The TNSS in both types of rhinitis changed significantly from the baseline during all follow-up periods (
Fig. 4): AR at 1 month (mean difference [95% CI]: 3.4981 [3.1207–3.8755]; I
2=0.0%), 3 months (3.1253 [2.7004–3.5501]; I
2=0.0%), 6 months (3.3284 [2.7670–3.8898]; I
2=9.5%), and 12 months (3.7090 [3.1356– 4.2823]; I
2=0.0%) (p=0.3853) and NAR at 1 month (3.2365 [2.3941–4.0789]; I
2=84.1%), 3 months (3.6008 [2.8051–4.3965]; I
2=71.4%), 6 months (3.9654 [2.2997–5.6311]; I
2=94.5%), and 12 months (4.8577 [4.4067–5.3087]; I
2=0.0%) (p=0.0017). Interestingly, the TNSSs in NAR increased significantly according to time (p=0.0017). Therefore, cryoablation would be effective in both types of rhinitis and have better long-term efficacy in NAR.
Fig. 3.
Changes in the TNSS subdomain after cryotherapy treatment for congestion (A), itching (B), rhinorrhea (C), and sneezing (D) [
3-
7,
9]. TNSS, total nasal symptom score.
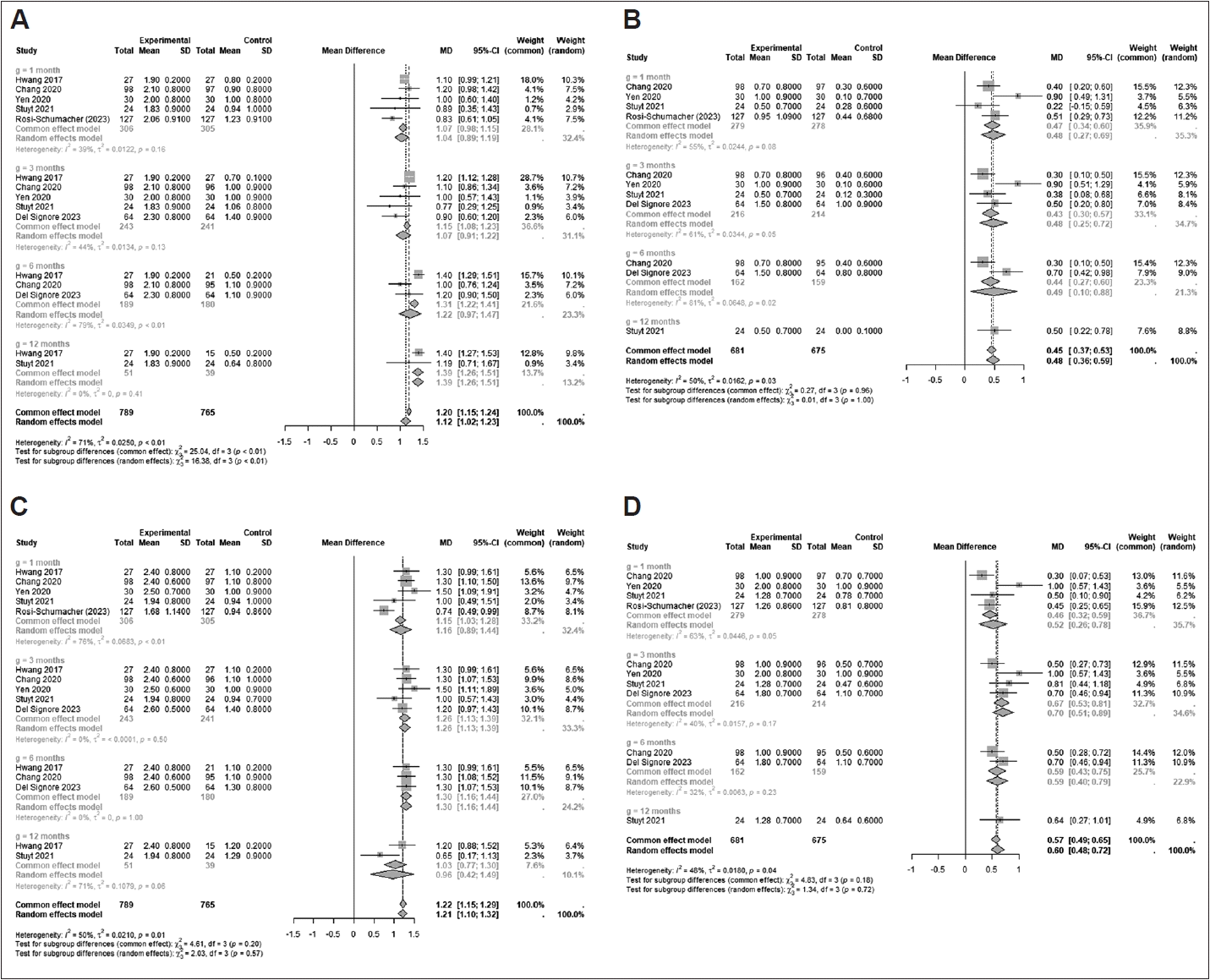

Fig. 4.
Changes in TNSSs after cryotherapy treatment for AR (A) and NAR (B) [
4,
6,
7,
9]. TNSS, total nasal symptom score; AR, allergic rhinitis; NAR, nonallergic rhinitis.





 PDF
PDF Citation
Citation Print
Print





 XML Download
XML Download