Introduction
The standard of care for individuals with severe hemophilia, as well as for some moderate hemophilia patients with a severe bleeding phenotype, involves prophylaxis, which is a regular replacement therapy designed to prevent bleeding and mitigate long-term consequences [
1]. Effective prevention and treatment of bleeding in hemophilia requires plasma factor activity to exceed a defined target level. However, considerable interpatient variability in the pharmacokinetics of factor concentrates suggests that relying on the average pharmacokinetic (PK) characteristics of such concentrates would not enable optimal treatment for individual patients [
2]. Additionally, this substantial interpatient variation in PK results in significant fluctuations in the required factor VIII (FVIII) dose to sustain the desired trough level for prophylaxis [
3].
Guidelines for pharmacokinetic studies involving FVIII concentrates recommend 10–11 blood samples over a period of 32–48 h (additional samples extending to 96 h or longer for the extended half-life of FVIII) [
1]. However, for dose tailoring in routine practice, useful PK parameters can be estimated using a smaller number of blood samples by leveraging population PK models using the Bayesian method [
4]. One notable tool is myPKFiT, a web-based device that employs the Bayesian model to estimate the PK curve of octocog alfa and rurioctocog alfa pegol (ADVATE
®; ADYNOVATE
®; Baxalta US Inc., a Takeda company, Lexington, MA, USA). The recommended times for blood sampling for PK simulation using myPKFiT, as stated in the myPKFiT Healthcare Professional User Manual, are first at 3−4 h (± 30 min) after infusion for both products, and the second at 24−32 h (± 1 h) for octocog alfa and at 48 h (± 2 h) and/or 72 h (± 2 h) for rurioctocog alfa pegol [
5]. This tool helps tailor prophylactic regimens for each patient [
6] and was approved by the Korean Ministry of Food and Drug Safety. This medical device is the most widely used population PK tool for patients with hemophilia A in South Korea.
In Korea, restrictions on the reimbursement of factor concentrates pose a challenge to the implementation of personalized treatments for patients with hemophilia [
7]. Moreover, Korean data regarding the PK of FVIII and its interpatient variability is limited. Understanding the PK profile of FVIII would be imperative for optimizing hemophilia treatment outcomes. Therefore, this study aimed to analyze the real-world PK of FVIII in Korean patients with hemophilia A.
Go to :

Methods
Study design, patients, and data collection
A retrospective chart review was conducted across five Korean hemophilia treatment centers located in Seoul, Daegu, Busan, and Gwangju. FVIII PK data were collected using myPKFiT between January 1, 2018, and November 30, 2021. We included patients with moderate to severe hemophilia A (baseline FVIII < 5 IU/dL) within the age range of 1–65 years for octocog alfa and 12–65 years for rurioctocog alfa pegol, who had PK test results at the commencement of the study. Patients with detectable levels of FVIII inhibitors (antibodies) at the time of PK testing, those diagnosed with congenital or acquired hemostatic disorders other than hemophilia A, and those enrolled in an interventional hemostatic trial during the PK testing period were excluded.
The primary objective was to describe the FVIII PK of octocog alfa and rurioctocog alfa pegol in Korean patients with hemophilia A. Differences in the PK profiles (halflife, time to 1%, and clearance) of octocog alfa and rurioctocog alfa pegol, controlled for age, blood type, inhibitor history, von Willebrand factor antigen (vWF:Ag) level, and body mass index (BMI) were also analyzed.
Statistical analysis
Data are presented as median (minimum, maximum), mean ± standard deviation (SD), or n (%). Group differences for each product were compared using parametric and non-parametric tests, as deemed appropriate. The Shapiro–Wilk test was applied to verify a normal distribution, and parametric tests were used to compare variables with a normal distribution. Parametric (independent t-test and ANOVA) or non-parametric (Mann–Whitney and Wilcoxon) methods were used for the analysis. BMI was derived from height and weight and categorized according to Korean guidelines. The association between the PK profile (half-life, time to 1%, and clearance) and age, BMI, and vWF:Ag was analyzed using a simple linear regression model. Age (< 6 years, ≥ 6– < 12 years, ≥ 12– < 18 years, and ≥ 18 years), blood type (O and non-O), and BMI (< 18.5 kg/m2, 18.5–22.9 kg/m2, 23.0–24.9 kg/m2, and ≥ 25.0 kg/m2) were treated as categorical variables.
Statistical significance was set at 5% (P < 0.05). Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) and R version 4.2.2 (R Foundation, Vienna, Austria).
Ethics statement
The study protocol and the associated case report forms (CRFs), were reviewed and approved by the Public Institutional Bioethics Committee of the Ministry of Health and Welfare (approval no. P01-202206–01-021).
Go to :

Discussion
Interpatient variations in the PK of FVIII have been reported in individuals with hemophilia. Based on these studies, various international organizations, such as the World Federation of Hemophilia (WFH), the United Kingdom Haemophilia Centre Doctors’ Organization (UKHCDO), and the National Bleeding Disorders Foundation (NBDF), recommend personalized prophylaxis that considers the unique PK profile of each patient [
1,
8,
9]. Consistent with this trend, this study established a basis for the implementation of PK-based personalized prophylaxis in Korea.
Consistent with our findings, several studies have reported wide variations in PK profiles. A simulation study, utilizing data from prospective clinical trials with octocog alfa, demonstrated a significant difference in time to 1% between patients in the 5th and 95th percentiles of half-lives, with a gap of 57.1 h (range: 46.4–103.5 h) [
3]. Another Canadian study presented a half-life range of 11.0–23.6 h in adolescent patients for rurioctocog alfa pegol [
10]. Similarly, the reported half-life values for a recombinant FVIII among Chinese pediatric patients showed a range of 7.1–22.6 h [
11]. However, real-world PK data for Asian patients, particularly for FVIII concentrates with extended half-lives, remains limited.
In addition, although the objective of this real-world study in Korean patients was not to directly compare the PK profiles of rurioctocog alfa pegol and octocog alfa, the half-life and time to 1% for rurioctocog alfa pegol were more than 1.5-fold longer than those of octocog alfa, whereas the clearance of rurioctocog alfa pegol was approximately 0.4 times-that of octocog alfa.
A linear relationship was observed between age and the PK of octocog alfa, which was characterized by an increase in half-life and time to 1%, along with a decrease in clearance. This finding aligns with a study conducted by Björkman, who also reported linear positive and negative correlations with the half-life and clearance of FVIII, respectively, based on age [
12]. However, in the case of rurioctocog alfa pegol, the present study did not observe statistically significant relationships between the PK parameters and age. This may be attributed to the exclusion of children under 12 years of age from the study owing to limitations in the PK simulation using myPKFiT.
Recovery of FVIII is increased in patients with a higher BMI because the increase in body weight is primarily due to increased adipose tissue, which contains less vascular space than lean mass. Consequently, obese individuals are likely to have a higher circulating plasma FVIII level [
13]. This study did not assess the recovery of FVIII; however, BMI showed a linear relationship with the clearance of rurioctocog alfa pegol. This result is consistent with the reported effects of BMI on clearance, as clinical trials have reported a negative correlation between BMI and clearance [
14,
15]. However, in the case of octocog alfa, the study did not find an association between BMI and clearance, which may be attributed to the inclusion of data from pediatric patients who have a larger volume of distribution than adults because of greater extracellular and total body water space. Additionally, metabolic rate, which varies with age, may also have an effect. In the BMI group analysis, the Korean BMI classification was used, which differs from that of the World Health Organization (
Table 6), and this distinction must be considered [
16,
17].
Table 6
BMI classifications (adults)
|
[unit: kg/m2] |
Underweight |
Normal |
Overweight |
Obesity |
|
Korea [16] |
< 18.5 |
18.5–22.9 |
23.0–24.9 |
≥ 25.0 |
|
WHO [17] |
< 18.5 |
18.5–24.9 |
25.0–29.9 |
≥ 30.0 |

This study also found a linear relationship between vWF:Ag and half-life, time to 1%, and clearance in rurioctocog alfa pegol, which is consistent with previous reports [
18–
20]. The relationship between vWF:Ag and blood type is known [
21], with non-blood type-O individuals having higher vWF:Ag levels than blood type-O individuals in patients with hemophilia [
22]. The PK analysis by blood type (O vs. non-blood type-O) revealed that the half-life and time to 1% were reduced in blood type-O and increased in non-blood type-O patients, whereas the opposite result was observed for the clearance of both products. Similar results have been reported in other studies [
14,
22,
23]. However, because of the small sample size (octocog alfa, 6/48; rurioctocog alfa pegol, 18/81), this study acknowledges that vWF:Ag, measured at an interval close to the PK test, may have failed to demonstrate a statistical correlation with the PK profile of octocog alfa.
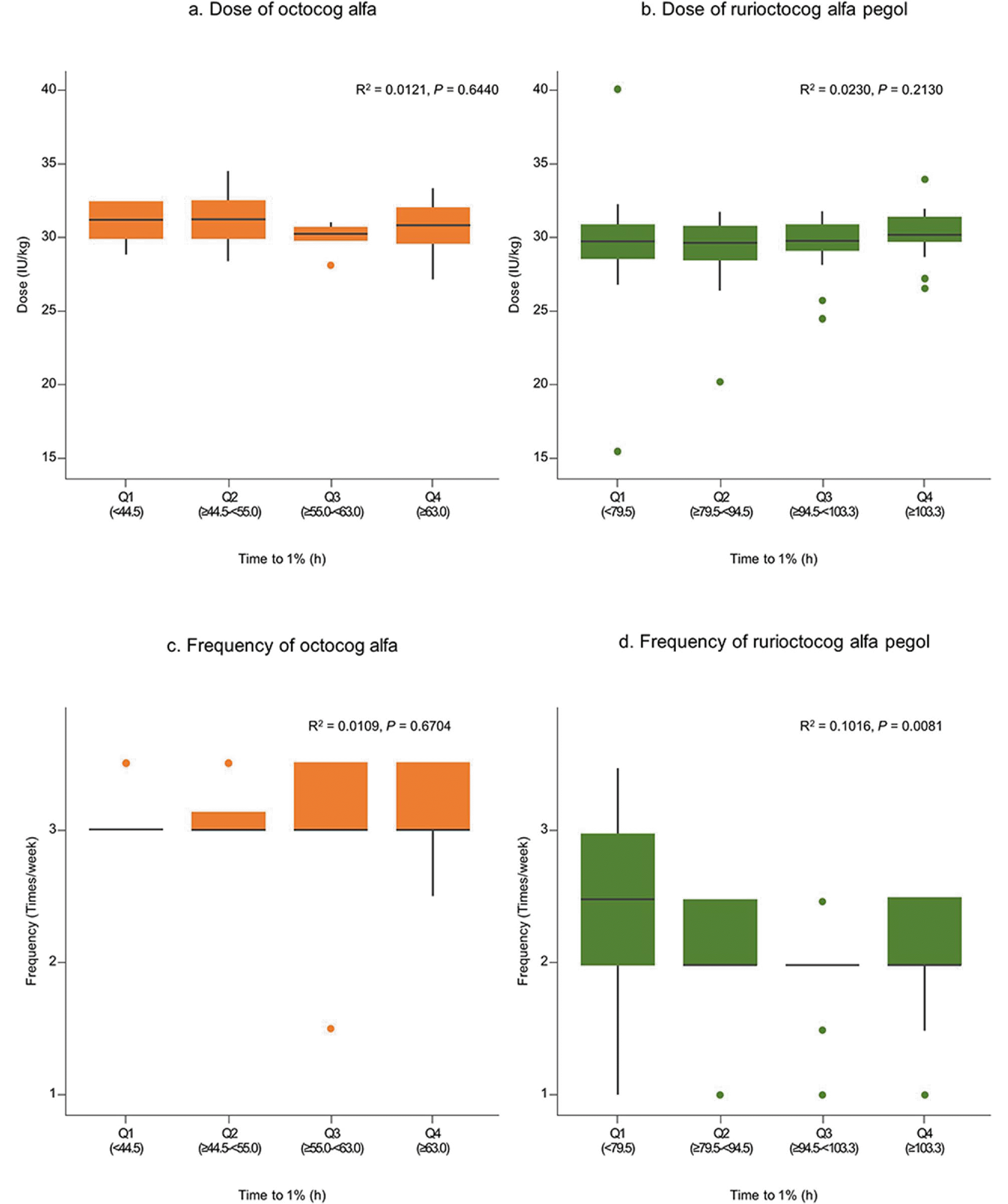
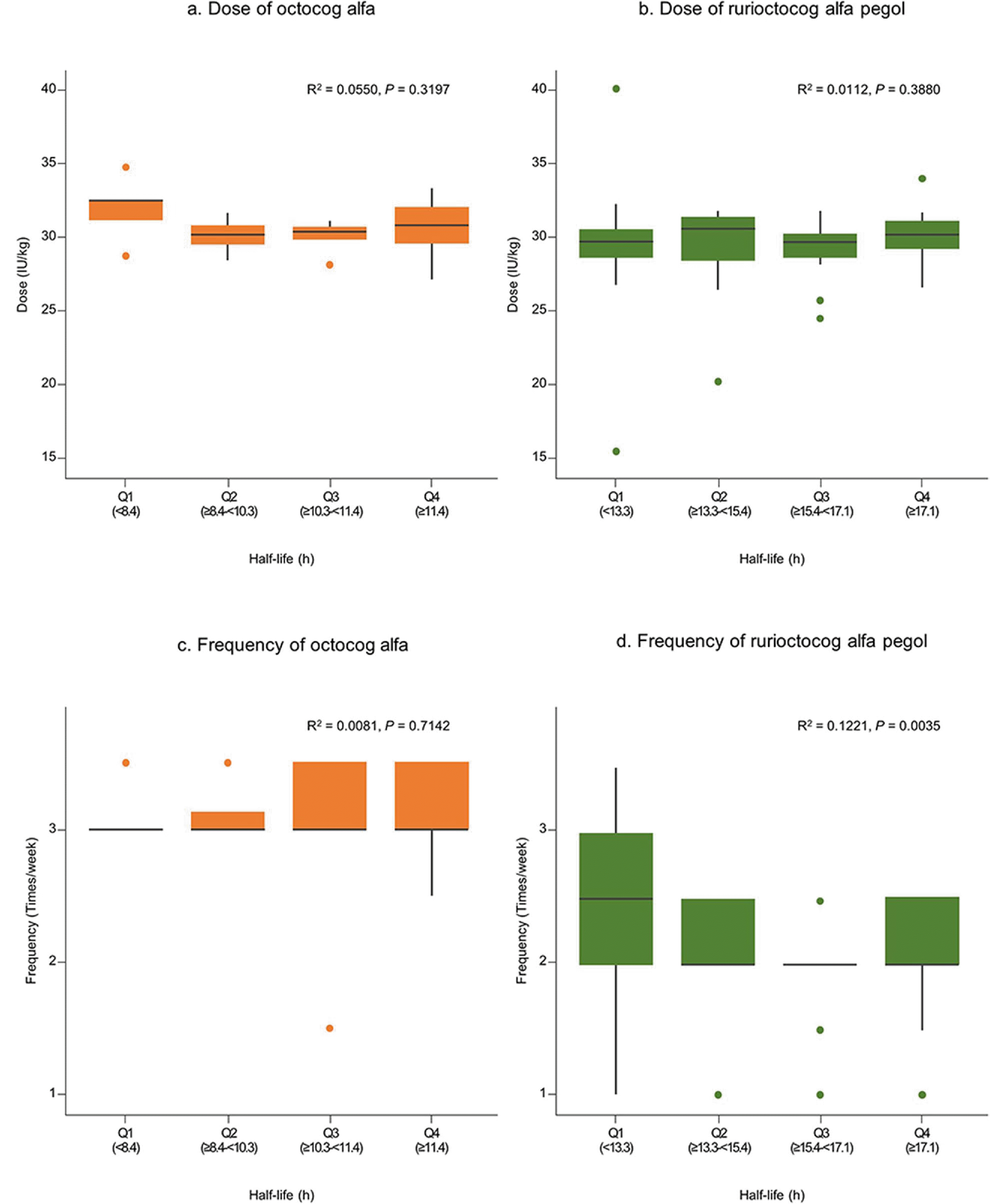
The dose and frequency by the PK parameters were analyzed in severe cases, which revealed no significant differences in patients stratified by quartiles of time to 1% and half-life. However, the frequency of rurioctocog alfa pegol showed a statistically significant difference based on time to 1% and half-life. Although statistically significant, the impact may not be meaningful because of the relatively small coefficients of determination (
R2 = 0.1016 for time to 1% and
R2 = 0.1221 for half-life). In this study, the mean dose and frequency of octocog alfa were 29.1 ± 4.7 IU/kg and 3.0 ± 1.5 times/week, respectively, while for rurioctocog alfa pegol, the mean dose and frequency were 28.8 ± 4.0 IU/kg and 2.2 ± 0.8 times/week, respectively. These values are very close to the upper limit of the reimbursement guidelines for patients with severe hemophilia A in Korea (up to 30 IU/kg, 3 times/week for standard half-life FVIII concentrates, such as octocog alfa, and 30 IU/kg, 2 times/week for extended half-life FVIII concentrates, such as rurioctocog alfa pegol) [
7].
Similar to other studies, this study revealed that the time to 1% and half-life of each product varied by more than 2–3 times among Korean patients. Personalized prophylaxis, which involves adjusting the dose and frequency based on individual PK profiles, is recommended for the optimal treatment of hemophilia. However, despite the observed PK variations among the patients, the prophylaxis dose and frequency of administration were close to the upper limit of reimbursement, regardless of the individual’s time to 1% or half-life. The limited flexibility to increase the dose or frequency is a cause for concern, particularly because PK tests are preferentially performed in patients with insufficient treatment outcomes under the current prophylaxis regimen.
A recent update to the reimbursement guidelines for FVIII concentrates permits increased doses in cases where the trough FVIII level is below 1% at the time points of 48 h and 72 h after the administration of standard and extended half-life FVIII concentrates, respectively [
24,
25]. This change is significant because it acknowledges the variations in individual PK. However, the 1% target trough FVIII level is considerably lower than the 3–5% recommended by the WFH. This discrepancy implies that patients still face a risk of bleeding [
1].
A few years ago, the WFH revised the definition of prophylaxis, emphasizing its purpose as a treatment that allows patients with hemophilia to perform physical and social activities akin to non-patients, prompting healthy and active lives [
1]. Therefore, there is a need for practices in Korea that reflect the interpatient variation observed in hemophilia. In addition to the PK-based personalized prophylactic regimen for patients with hemophilia A, considering individual and situation-specific dosing schedules to maintain an appropriate factor level according to the time of physical and social activity is important. The use of a patient application with real-time FVIII level estimation and patient education to properly utilize this information are also recommended.
To the best of our knowledge, this is the only real-world study analyzing the PK of FVIII in Korean patients, including a substantial number of patients receiving FVIII with an extended half-life. However, this study had some limitations; for example, children under 12 years of age could not be enrolled in the rurioctocog alfa pegol study because PK simulation with myPKFiT cannot be performed in this age group. Missing data, particularly concerning the vWF:Ag level, were attributed to the retrospective nature of this study. Additionally, the number of patients with a history of inhibitor titers of 0.6 BU or higher was small, limiting the analysis of the correlation with the PK profile. Future studies should focus on regimen changes based on individual PK profiles and treatment outcomes.
This study underscores the significant interpatient variation in the PK of FVIII concentrates in Korean patients with hemophilia A. To optimize the effectiveness of prophylactic regimens and improve the overall treatment outcomes, a personalized approach must be implemented and tailored according to an individual patient’s PK profile. Moreover, treatment strategies should target the prevention of bleeding and enable healthy and active lifestyles in patients with hemophilia.
Go to :





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download