Abstract
Background
Modern neurosurgery has undergone significant evolution to include minimally invasive procedures, with the supraorbital approach (SOA) being a prime example. In this study, we aim to explore the surgical techniques and outcomes of this approach in the surgical treatment of frontal lobe, anterior skull base, and parasellar lesions.
Methods
This study included 33 patients aged 36–83 years who underwent surgery using the SOA for lesions in the inferior frontal lobe, anterior skull base, and parasellar area between 2015 and 2024. There were 25 cases of meningioma, 2 cases of brain abscess, 2 cases of glioma, and one case each of craniopharyngioma, hemangioma, metastasis, and Rathke’s cleft cyst. The medical data and follow-up results were retrospectively analyzed.
Results
The mean size of lesion was 3.38±3.05 cm. The mean follow-up period was 48.8 months. Gross total resection was achieved in 25 patients (75.8%). There were no perioperative deaths, cases of cerebrospinal fluid rhinorrhea, or infections. Two cases of morbidity were reported as complications: one case of delayed intracerebral hemorrhage and one case of infarction due to vascular injury. All patients exhibited satisfactory cosmetic results.
Conclusion
In comparison to the conventional pterional approach, the SOA represents a safe and effective keyhole method for the removal of both extra-axial and intra-axial skull base tumors. This is particularly beneficial for lesions in the orbitofrontal region and parasellar area, as it allows for minimal disruption of normal brain parenchyma. Moreover, the SOA promotes a swift recovery and short hospital stay. Additionally, the SOA yields superior cosmetic results, including the prevention of temporalis muscle atrophy.
The traditional surgical approaches for brain tumors occurring in the anterior skull base region have involved extensive skin and skull incisions, such as the pterional approach, subfrontal approach, and orbitozygomatic approach [12]. These methods, performed through long skin incisions and large cranial openings, often lead to significant damage to surrounding tissues and complications. In contemporary neurosurgery, advancements in surgical microscopy, endoscopy, and navigation devices have allowed for the safe removal of lesions through smaller craniotomy [345].
The supraorbital approach (SOA), introduced by Fedor Krause in 1908, is an example of a minimally invasive surgical technique that has been widely utilized since its inception [67]. This approach, involving a small eyebrow skin incision, allows for the safe removal of lesions with reduced damage to surrounding tissues. This study outlines our clinical observations based on 33 consecutive patients who underwent a minimally invasive supraorbital keyhole craniotomy with an incision in the eyebrow region.
As our knowledge repository expands through the integration of recently refined methodologies, it is prudent to engage in a thorough examination of extensive datasets. This approach allows for a discerning retrospective analysis to extract and refine the insights gained from this accumulated experience.
Thirty-three patients (11 males: 33.3% and 22 females: 66.7%) with anterior skull base lesions, inferior frontal lobe, and parasellar lesions were treated at our institution using the SOA between January 2015 and January 2024. This study was conducted retrospectively. This study was approved by the Institutional Review Board of Soonchunhyang University Seoul Hospital, but informed consent was waived because of the retrospective nature of the study and the analysis used anonymous clinical data (IRB No. 2024-04-005).
All patient underwent preoperative and postoperative clinical and radiologic evaluations. Among the 33 patients treated with the SOA, there were 25 cases of meningioma, 2 cases of brain abscess, 2 cases of glioma, and one case each of craniopharyngioma, hemangioma, metastasis, and Rathke’s cleft cyst (Table 1). Among a total of 25 patients with meningiomas, 8 had tuberculum sellae meningiomas, 7 had olfactory groove meningiomas, 6 had clinoid meningiomas, and 4 had frontal convexity meningiomas. Postoperative imaging follow-up was performed using gadolinium-enhanced brain MRI. The initial MRI was conducted six months after surgery, with subsequent examinations scheduled every one to two years.
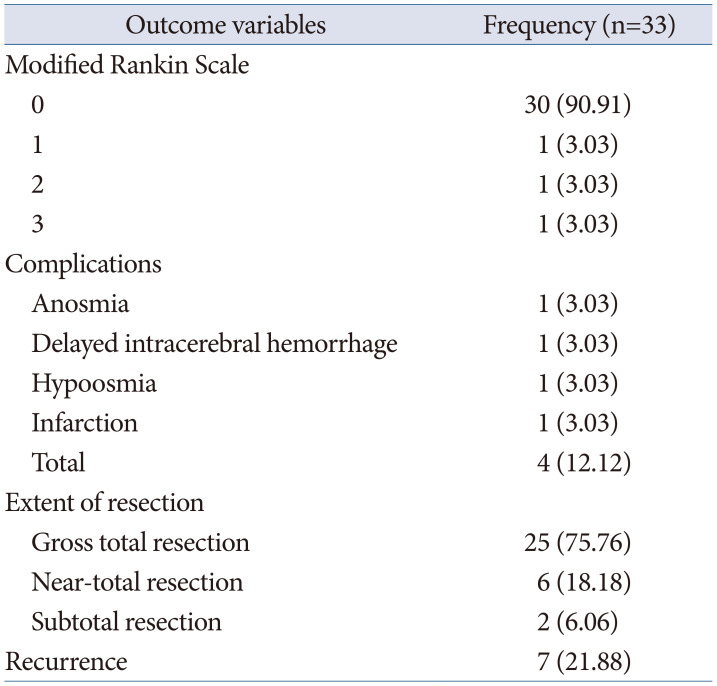
Postoperative surgical outcomes were evaluated using the Modified Rankin Scale, with assessments including the number of days of hospital stay, extent of tumor removal, complications, and recurrence.
Under general anesthesia, after endotracheal intubation and insertion of a urinary catheter, the head is positioned at approximately 15–20 degrees of flexion using either the Mayfield head fixation device or a horseshoe headrest. While head extension is commonly used in general SOA procedures, our experience has shown that a head flexion of approximately 15–20 degrees can optimize the surgical field and facilitate access to the surgical site. In particular, a slight head flexion can make the use of surgical instruments more convenient when approaching the frontal lobe or the skull base. It can be beneficial to individualize head positioning based on the surgical approach and the patient’s anatomical structure. The degree of head rotation, ranging from 15 to 60 degrees in the opposite direction, depends on the tumor’s location. For tumors situated anteriorly in the central region, such as olfactory groove meningioma (OGM), head rotation may be extended up to 60 degrees.
During the skin incision, particular emphasis is placed on preventing sensory deficits in the forehead that may arise from post-surgery damage to the supraorbital nerve. It is recommended to incise the skin along the upper part of the eyebrow, at least 5 mm away from the supraorbital foramen, promoting a location beyond this point. If a larger bone flap is required, the skin incision can be extended along the orbital rim. When performing a skin incision on the eyebrow, it is not necessary to shave the eyebrows. It has been reported that not shaving the eyebrows does not pose a risk of infection. Additionally, there is an advantage in that post-surgery scars are not prominently visible.
The skin incision is performed using a No. 15 blade, extending through the dermal layer. Subsequently, the incision progresses to include the upper frontalis muscle above the orbicularis oculi muscle, with partial incision of the temporalis muscle. Care is taken to avoid damage to the orbicularis oculi muscle during this procedure.
Traction of the skin and muscles is performed using fish hooks to minimize traction injury. A 5 mm burr hole is made in the temporal bone corresponding to MacCarty’s keyhole, followed by a 1.2–2 × 2.5–4 cm bone flap creation (Fig. 1).
When dealing with a large frontal sinus, the bone flap should be created from a more lateral direction to avoid the frontal sinus, reducing the risk of postoperative cerebrospinal fluid leakage and infection. In cases where preoperative imaging modalities such as CT or MRI reveal an increased size of the frontal air sinus with a potential risk of exposure during operative procedures, the using of a periosteal flap is viable to securely seal any resultant open cavities (Fig. 1).
Extreme caution is needed during the dissection of the temporalis muscle to prevent damage to the frontal branches of the facial nerve. If the frontal sinus is exposed, it should be well sealed with bone wax or betadine-soaked gel foam.
To minimize frontal lobe retraction, the dura mater is dissected off the orbital roof and then flattened using a high-speed drill. Care should be taken to avoid damage to the periorbita, as damage may lead to orbital fat herniation. Subsequently, a C-shaped dural incision is made, exposing the frontal lobe and temporal lobe, which is gently retracted using a brain retractor. The dura is then opened, and cerebrospinal fluid is drained. Further steps, such as Sylvian fissure dissection or optic nerve decompression, can be performed if necessary.
After removing the tumor using tools such as the cavitron ultrasonic aspirator and bipolar coagulator, the dura mater is sutured with a 4-0 Nurolon suture (Ethicon, NJ, USA), and the bone flap is secured using a titanium mesh.
The frontal muscle must be sutured, and the skin can be closed with subcuticular sutures or 6-0 nylon sutures, with the option of using sterile strips for wound closure.
A total of 33 patients (11 males and 22 females) were included in this study. The patients ranged in age from 36 to 83 years, with an mean age of 61.67±11.56 years (Table 1). The mean follow-up period was 48.8 months (range 3–108 months).
The lesions were most commonly located on the midline in 16 (48.5%) patients, followed by 10 (30.3%) on the right side and 7 (21.2%) on the left side. The mean tumor size was 3.38±3.05 cm (range 1.0–4.7 cm) (Table 2).
Preoperative symptoms included headache and visual impairment (decreased visual acuity/visual field defect), each affecting 10 (30.3%) patients, making them the most common symptoms. Cognitive impairment was observed in 2 patients, while dysarthria, gait disturbance, ptosis, memory disturbance, and syncope were each observed in 1 patient. Additionally, 5 cases were incidentally discovered without any symptoms (Table 3).
Gross total resection was achieved in 25 cases (75.8%), near-total resection in 6 cases (18.2%), and subtotal resection in 2 cases (6.0%). In the four cases where gross total resection was not achieved for meningiomas, three cases involved tumor invasion into the cavernous sinus. The remaining case was characterized by severe adhesion and fibrosis with the optic nerve, resulting in a portion of the tumor being left behind. In cases of metastatic brain tumors and anaplastic oligodendrogliomas, near-total resection and subtotal resection were performed, respectively (Table 4).
For the two cases of meningiomas with cavernous sinus invasion, CyberKnife radiosurgery was additionally performed on the remaining tumors, and to date, the tumors have been controlled.
Complications occurred in four cases (12.1%): one case of clinoid meningioma experienced delayed intracerebral hemorrhage (ICH), and one case of olfactory groove meningioma suffered a cerebral infarction due to injury to an anterior cerebral artery branch during surgery. In these two cases, the surgical outcomes on the modified Rankin Scale were scored as 1 and 2, respectively, while in the remaining 29 cases, all were scored as 0. Among the seven patients with olfactory groove meningiomas, complications occurred in two individuals: one experienced hypoosmia, and the other developed anosmia (Table 4). In the eight cases of tuberculum sella meningiomas, there were fortunately no instances of postoperative visual deterioration compared to preoperative levels.
Excluding cases with major complications or those requiring extended hospitalization for additional radiotherapy and other treatments, the average length of stay was 11.4 days. Postoperatively, the appearance of the surgical wound was satisfactory to patients in all cases.
A 42-year-old woman presented with a gradual decline in vision. Ophthalmological examination revealed near-total visual field loss in the right eye, except for preservation within the central 5 degrees, and visual field deficits involving approximately 3/4 of the left eye, excluding the nasal periphery. Brain MRI revealed a uniformly enhancing extra-axial tumor measuring 4.1×3.1 cm, originating from the olfactory groove, extending up to the superior aspect of the sella turcica. Compression of both optic nerves and the optic chiasm was observed (Fig. 2B and C). A meningotheliomatous meningioma, corresponding to World Health Organization (WHO) grade 1, was diagnosed. Surgical resection via a right SOA was performed under general anesthesia, achieving a Simpson grade 2 resection (Fig. 2D and 3). Postoperatively, there was improvement in vision and visual fields, and at 6 months, the surgical scar was minimally noticeable to the naked eye (Fig. 4).
A 51-year-old man presented with decreased vision in the right eye. The patient has a broad-based extra-axial enhancing lesion, measuring approximately 3.6×2.1×3.1 cm, primarily located on the right side of the anterior cranial fossa. The tumor adhering to the right optic nerve was closely attached to the optic nerve, posing a risk of nerve damage during surgery (Fig. 5). This lesion involves the tuberculum sellae to the sella and extends to the right sphenoid ridge, with encasement of the right orbital apex (Fig. 6A-C).
Therefore, a portion of the tumor was left in place. In the postoperative MRI, the tumor was mostly well removed, with only a small portion remaining on the optic nerve located at the orbital apex (Fig. 6D-F). Histopathological examination confirmed the diagnosis of transitional and psammomatous meningioma. The patient showed a favorable postoperative course.
Surgical treatment for tumors occurring in the anterior skull base, frontal base and periorbital regions traditionally involved large skin and skull incisions, such as the frontotemporal or subfrontal approaches, to widen the surgical field [189]. However, larger craniotomies are associated with increased risks of complications, including infection, bleeding, temporal muscle atrophy, hair loss at the incision site, and sensory deficits.
Recent advancements in radiological imaging, such as brain CT and MRI, along with the development of surgical techniques and intraoperative monitoring devices, have enabled precise delineation of tumors and surrounding structures. With these advancements, even with minimal craniotomy, accessing the base of the brain for tumor removal has become feasible. Tumors in the frontal and periorbital regions can now be approached with less extensive bone removal, thanks to improved visualization using neuroendoscopy and microscopic techniques [1011].
Tumors in these regions include OGMs, planum sphenoidale meningiomas, tuberculum sellae meningiomas (TSM), clinoidal meningiomas, cavernous hemangiomas, craniopharyngiomas, pituitary adenomas, metastatic brain tumors, gliomas, and brain abscesses [4512131415].
For OGMs, the SOA allows for minimal frontal lobe retraction while providing a clear view of the tumor. In the case of an OGM, even if the size exceeds a diameter of 3 cm, since the tumor has already created space, performing internal decompression first can allow for the removal of the tumor without the need for extensive brain retraction [16]. Surgical intervention on seven patients with OGMs was conducted using the SOA. In three of these cases, the tumors extended to the crista galli. Adequate tumor removal was facilitated by rotating the head by approximately 60 degrees in these instances (Fig. 7).
This approach minimizes the risk of optic nerve injury, especially when dealing with TSM. The current literature on the surgical management of TSM underemphasizes the incidence of optic canal involvement. Initiating the surgical procedure by opening the falciform ligament and the optic nerve sheath proves beneficial in safeguarding the optic nerve while extracting a tumor situated within the optic canal [17]. In cases of TSM, the optic nerve situated on the opposite side is more visible, and the dissection of the tumor from the optic nerve is facilitated compared to the same side. Therefore, approaching from the side opposite to the tumor’s location may be more beneficial for the protection of the optic nerve [1819]. In cases involving optic canal invasion, the SOA can offer several advantages over the conventional pterional approach. SOA generally provides a wider field of view, facilitating access to the optic canal and surrounding structures. This is particularly beneficial when the optic canal is involved, as it allows for more precise and safer tumor removal. Additionally, SOA is relatively less invasive, which minimizes damage to the surrounding brain tissue and blood vessels, promoting faster recovery and reducing the risk of complications. Furthermore, the SOA allows for better visualization and protection of the optic nerve. Opening the falciform ligament and the optic nerve sheath first helps in minimizing optic nerve injury while removing the tumor. Using SOA also makes it possible to approach the tumor from the opposite side, which can be advantageous for better visualization and protection of the optic nerve. Approaching from the opposite side allows for easier dissection of the tumor with less interference to the optic nerve.
When removing a tumor via a SOA, there are instances where the feeding artery to the tumor branches off from the anterior cerebral artery or the middle cerebral artery, leading to bleeding. Due to the limited space, suturing is often not feasible; thus, a mini-hemoclip can be utilized to seal the small vascular opening, allowing for the control of bleeding (Fig. 8).
In instances where the tumor is situated at the temporal pole, the skin incision can be extended an additional 1–2 cm along the lateral orbital rim. Following this, further drilling of the lesser wing of the sphenoid bone is performed until the temporal dura is exposed, facilitating the removal of the tumor (Fig. 9). In our SOA series, we encountered a case involving a 59-year-old female patient with a meningioma located at the temporal pole. Preoperative and postoperative MRI confirmed that the tumor was completely removed (Fig. 10).
Overall, the SOA offers several advantages, including reduced surgical time, minimal bleeding, no need for head fixation devices, less postoperative pain, minimal temporal muscle atrophy, and less noticeable scarring [121520]. It allows for effective removal of tumors, even those invading the optic nerve or internal carotid artery, while preserving important structures. However, it requires expertise, and care must be taken to avoid unexpected vascular injuries or manage severe brain swelling effectively.
While the SOA can be highly effective for certain skull base tumors, its limitations must be carefully considered when planning the surgical approach. First, the SOA may provide a more limited exposure compared to other approaches like the pterional or orbitozygomatic approaches. This can be a challenge when dealing with larger or more complex tumors that extend beyond the reach of the SOA. Second, the narrow corridor of the SOA can restrict the maneuverability of surgical instruments, making it more challenging to perform intricate dissections or to handle complications that may arise during surgery. Third, the proximity to the frontal lobe increases the risk of brain retraction injury, which can lead to postoperative cognitive or behavioral changes. Also, the SOA requires specialized skills and experience, and there is a steep learning curve for surgeons to perform this approach effectively and safely. The choice of approach should be individualized based on the specific characteristics of the tumor and the patient’s anatomy.
In conclusion, the SOA is a minimally invasive surgical technique with benefits such as shorter surgery duration, reduced bleeding, minimal postoperative pain, and inconspicuous scarring. However, it requires proficiency, especially in handling unexpected complications, making it a valuable option for experienced surgeons in the treatment of tumors in the anterior skull base, frontal base, and periorbital regions.
In conclusion, SOA for surgeries targeting tumors on the anterior skull base, inferior frontal lobe, and parasellar region is a less invasive method when compared to traditional approaches. This technique has been proven safe and effective in removing both extrinsic and intrinsic tumors. It has also been effectively used to remove tumors at the temporal pole, demonstrating its effectiveness and adaptability. Consequently, the SOA is considered a viable minimally invasive alternative to the pterional approach for lesions that are located at the midline base or are laterally displaced but still proximate to the frontal base.
Moreover, the SOA has the advantage of preventing postoperative atrophy of the temporalis muscle, thereby offering better cosmetic outcomes than traditional surgical methods.
Notes
Author Contributions:
Conceptualization: Sung Jin Cho.
Data curation: Han Gyul Lee.
Formal analysis: Hye Ran Park.
Investigation: Dongwook Seo.
Methodology: Sung Jin Cho.
Project administration: Sung Jin Cho.
Resource: Hye Ran Park.
Software: Han Gyul Lee.
Supervision: Sung Jin Cho.
Validation: Hye Ran Park.
Visualization: Han Gyul Lee.
Writing—original: Han Gyul Lee, Sung Jin Cho.
Writing—review & editing: all authors.
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
References
1. Adappa ND, Lee JY, Chiu AG, Palmer JN. Olfactory groove meningioma. Otolaryngol Clin North Am. 2011; 44:965–980. PMID: 21819883.
2. Rychen J, Croci D, Roethlisberger M, Nossek E, Potts M, Radovanovic I, et al. Minimally invasive alternative approaches to pterional craniotomy: a systematic review of the literature. World Neurosurg. 2018; 113:163–179. PMID: 29452317.
3. Gazzeri R, Nishiyama Y, Teo C. Endoscopic supraorbital eyebrow approach for the surgical treatment of extraaxialand intraaxial tumors. Neurosurg Focus. 2014; 37:E20.
4. Robinow ZM, Peterson C, Waldau B, Shahlaie K. Supraorbital keyhole craniotomy via eyebrow incision: a systematic review and meta-analysis. World Neurosurg. 2022; 158:e509–e542. PMID: 34775096.
5. Youngerman BE, Shtayer L, Gerges MM, Larsen AG, Tomasiewicz HC, Schwartz TH. Eyebrow supraorbital keyhole craniotomy for olfactory groove meningiomas with endoscope assistance: case series and systematic review of extent of resection, quantification of postoperative frontal lobe injury, anosmia, and recurrence. Acta Neurochir (Wien). 2021; 163:101–112. PMID: 32888076.
6. Reisch R, Perneczky A, Filippi R. Surgical technique of the supraorbital key-hole craniotomy. Surg Neurol. 2003; 59:223–227. PMID: 12681560.
7. Zumofen DW, Rychen J, Roethlisberger M, Taub E, Kalbermatten D, Nossek E, et al. A review of the literature on the transciliary supraorbital keyhole approach. World Neurosurg. 2017; 98:614–624. PMID: 27989977.
8. Pepper JP, Hecht SL, Gebarski SS, Lin EM, Sullivan SE, Marentette LJ. Olfactory groove meningioma: discussion of clinical presentation and surgical outcomes following excision via the subcranial approach. Laryngoscope. 2011; 121:2282–2289. PMID: 21994142.
9. Sade B, Lee JH. High incidence of optic canal involvement in tuberculum sellae meningiomas: rationale for aggressive skull base approach. Surg Neurol. 2009; 72:118–123. PMID: 19147207.
10. Chen HC, Tzaan WC. Microsurgical supraorbital keyhole approach to the anterior cranial base. J Clin Neurosci. 2010; 17:1510–1514. PMID: 20817469.
11. Tatarli N, Ceylan D, Şeker A, Solmaz B, Çavdar S, Kiliç T. The supraorbital keyhole approach. J Craniofac Surg. 2015; 26:1663–1667. PMID: 26114521.
12. Eroglu U, Shah K, Bozkurt M, Kahilogullari G, Yakar F, Dogan İ, et al. Supraorbital keyhole approach: lessons learned from 106 operative cases. World Neurosurg. 2019; 124:e667–e674. PMID: 30659969.
13. Iacoangeli M, Nocchi N, Nasi D, DI Rienzo A, Dobran M, Gladi M, et al. Minimally invasive supraorbital key-hole approach for the treatment of anterior cranial fossa meningiomas. Neurol Med Chir (Tokyo). 2016; 56:180–185. PMID: 26804334.
14. Wiedemayer H, Sandalcioglu IE, Wiedemayer H, Stolke D. The supraorbital keyhole approach via an eyebrow incision for resection of tumors around the sella and the anterior skull base. Minim Invasive Neurosurg. 2004; 47:221–225. PMID: 15346318.
15. Wilson DA, Duong H, Teo C, Kelly DF. The supraorbital endoscopic approach for tumors. World Neurosurg. 2014; 82:e243–e256. PMID: 23395805.
16. Bander ED, Pandey A, Yan J, Giantini-Larsen AM, Schwartz A, Estin J, et al. Olfactory groove meningiomas: supraorbital keyhole versus orbitofrontal, frontotemporal, or bifrontal approaches. J Neurosurg. 2023; 140:1568–1575. PMID: 38064694.
17. Cai M, Hou B, Luo L, Zhang B, Guo Y. Trans-eyebrow supraorbital keyhole approach to tuberculum sellae meningiomas: a series of 30 cases with long-term visual outcomes and recurrence rates. J Neurooncol. 2019; 142:545–555. PMID: 30796744.
18. Singh H, Essayed WI, Jada A, Moussazadeh N, Dhandapani S, Rote S, et al. Contralateral supraorbital keyhole approach to medial optic nerve lesions: an anatomoclinical study. J Neurosurg. 2017; 126:940–944. PMID: 27257841.
19. Troude L, Boucekine M, Baucher G, Farah K, Boissonneau S, Fuentes S, et al. Ipsilateral vs controlateral approach in tuberculum sellae meningiomas surgery: a retrospective comparative study. Neurosurg Rev. 2021; 44:3581–3591. PMID: 33890190.
20. Beseoglu K, Lodes S, Stummer W, Steiger HJ, Hänggi D. The transorbital keyhole approach: early and long-term outcome analysis of approach-related morbidity and cosmetic results. J Neurosurg. 2011; 114:852–856. PMID: 21029037.
Fig. 1
Intraoperative close-up of MacCarty’s keyhole. A: Intraoperative close-up of MacCarty’s keyhole after reflection of periosteal flap and anterior portion of temporlis muscle. B: Removal of the bone flap exposes the dura mater.
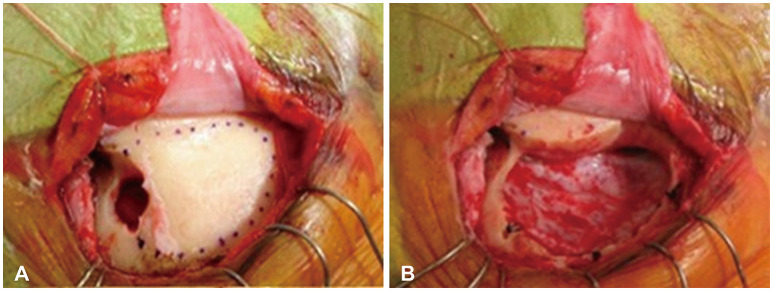
Fig. 2
Intraoperative microscopic images with a surgical microscope during surgery on olfactory groove meningioma using the supraorbital approach approach. A: C-shape durotomy was made after supraorbital craniotomy. B and C: Exposure of a tumor (T) mass after frontal lobe retraction. D: After removal of tumor, exposure of optic nerve (ON), anterior cerebral artery (A1), and middle cerebral artery (M1).
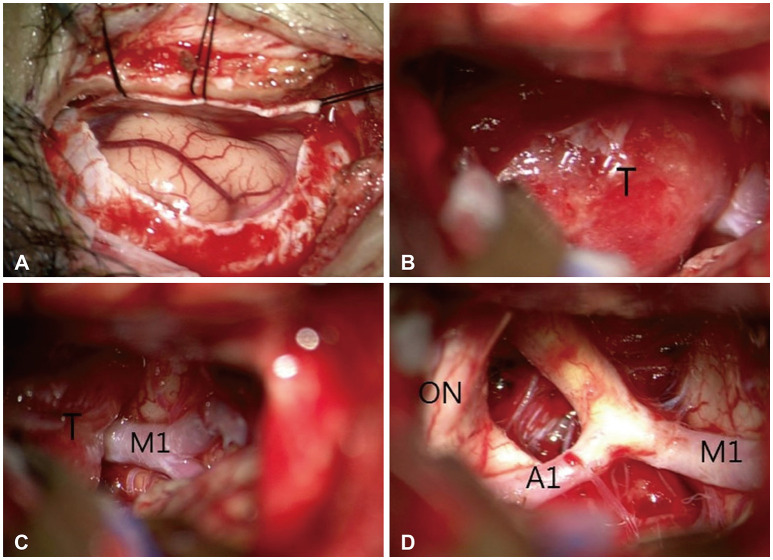
Fig. 3
Preoperative MRI, including axial (A), coronal (B), and sagittal (C) views, demonstrates a homogeneously enhancing large frontal-based tumor measuring 4.1×3.1 cm with suprasellar extension, suggestive of an olfactory groove meningioma. Postoperative MRI, including axial (D), coronal (E), and sagittal (F) views, demonstrate complete tumor removal.
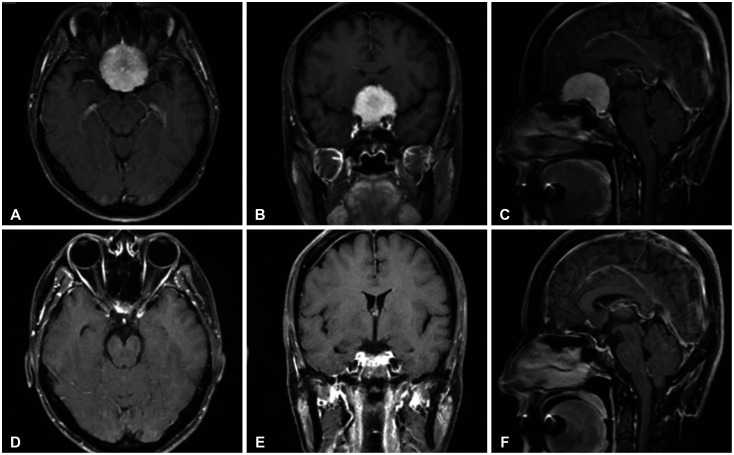
Fig. 5
Intraoperative surgical images. A: To enhance exposure of the temporal area, the skin incision was extended from the right eyebrow to the lateral orbital rim, avoiding the supraorbital nerve (black arrow: supraorbital nerve direction). B: The image depicts the skull bone exposed following a skin incision. C: After opening the dura, the proximal Sylvian fissure was opened to minimize brain retraction. D: After tumor removal, the tumor was found to be tightly adhered to the right optic nerve. The tumor at the temporal pole was also adequately excised. Arrow, supraorbital notch; F, frontal; T, temporal; Tm, tumor; SV, Sylvian vein; LON, left optic nerve; RON, right optic nerve; ICA, internal carotid artery.
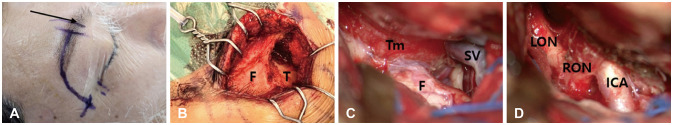
Fig. 6
Pre- and postoperative MRI images. A-C: Preoperative MRI images; A broad-based extra-axial enhancing lesion, measuring approximately 3.6×2.1×3.1 cm, is primarily located on the right side of the anterior cranial fossa, involving the planum sphenoidale through the tuberculum sella and extending to the right sphenoid ridge, with encasement of the right orbital apex. D-F: The MRI images post-tumor removal show that the tumor located at the sphenoid ridge has also been excised.
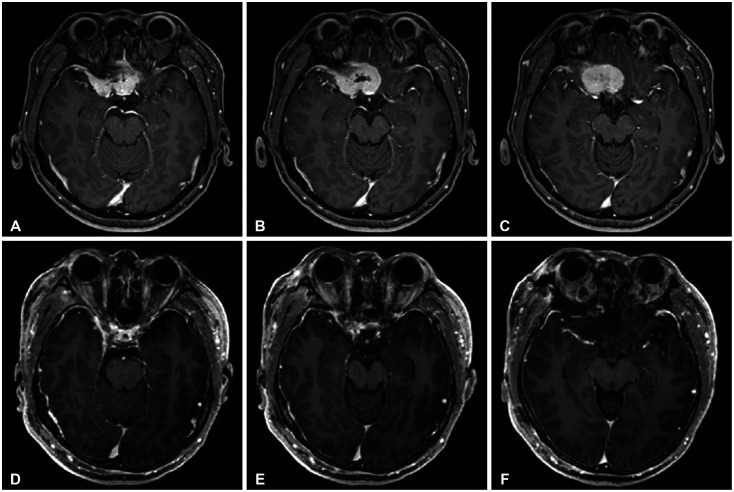
Fig. 7
Representative preoperative sagittal contrast-enhanced T1-weighted MR images obtained in 7 patients with olfactory groove meningiomas included in this study (upper) and 3 cases overlying the cribriform plate and anterior to the insertion of the sphenoid sinus. Lower images indicate MRI images after the removal of a tumor.
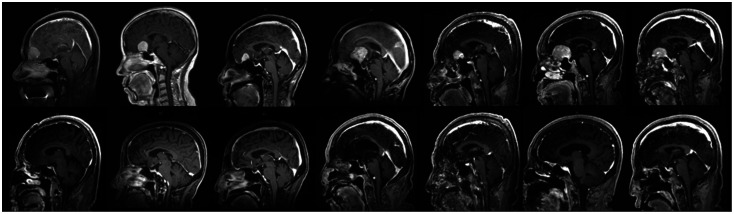
Fig. 8
This image depicts the control of arterial bleeding with mini-hemoclips, which was caused by cutting the feeding artery from the anterior cerebral artery to the tumor during the removal of an olfactory groove meningioma.
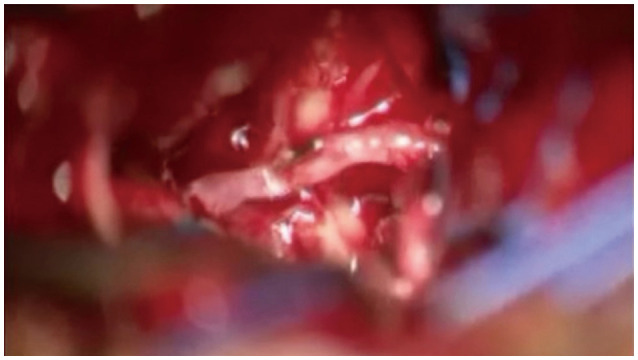
Fig. 9
Intraoperative surgical images for temporal pole meningioma. A: The image illustrates that, following the removal of the frontal bone, the frontal dura was exposed utilizing a right supraorbital approach. To expose the temporal pole, additional drilling of the sphenoid ridge (SR) is necessary. B: After the removal of the sphenoid ridge, the temporal dura was additionally exposed. C: This image demonstrates that following the resection of the tumor, the tumor located at the temporal pole was completely excised. F, frontal; T, temporal; SOR, superior orbital rim; SR, sphenoid ridge.
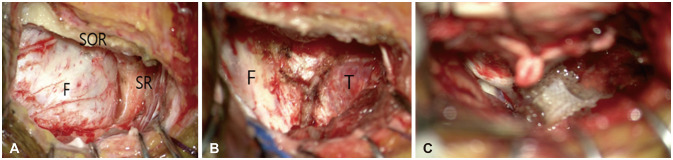
Fig. 10
Preoperative MRI images (A-D) show a homogeneously enhancing mass measuring approximately 2.9×2.4×2.2 cm in the right temporal convexity. Postoperative MRI images (E-H) show complete tumor removal using the supraorbital approach.
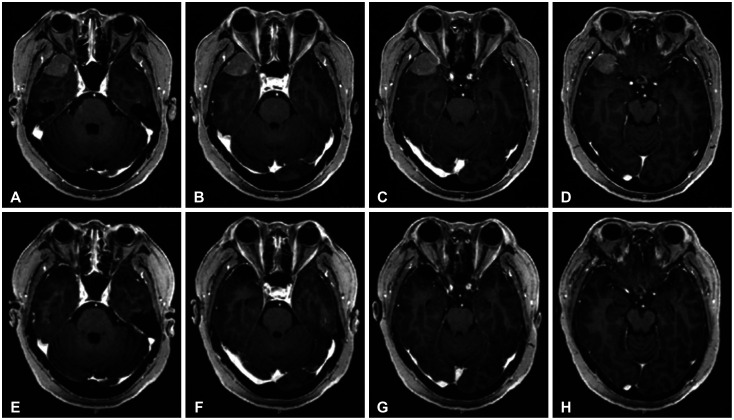
Table 1
Characteristics of patients who underwent surgery using the supraorbital approach
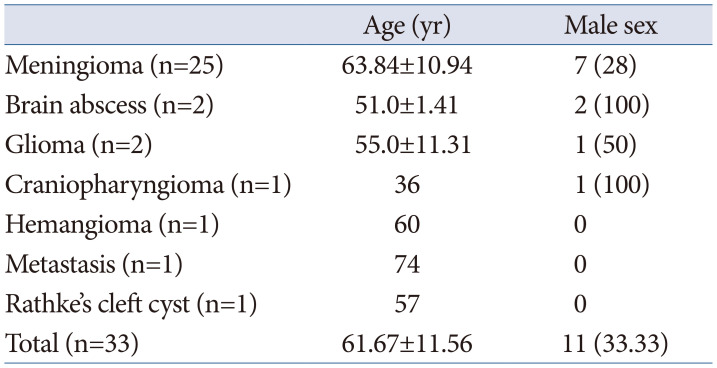
Table 2
Tumor characteristics
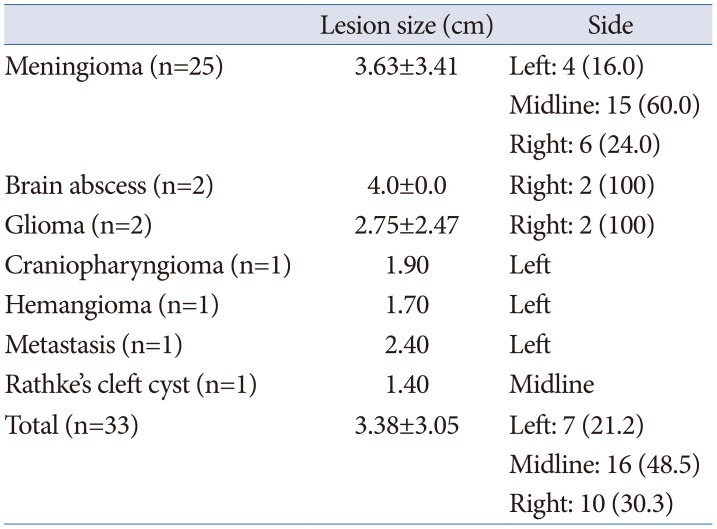
Table 3
Preoperative symptoms
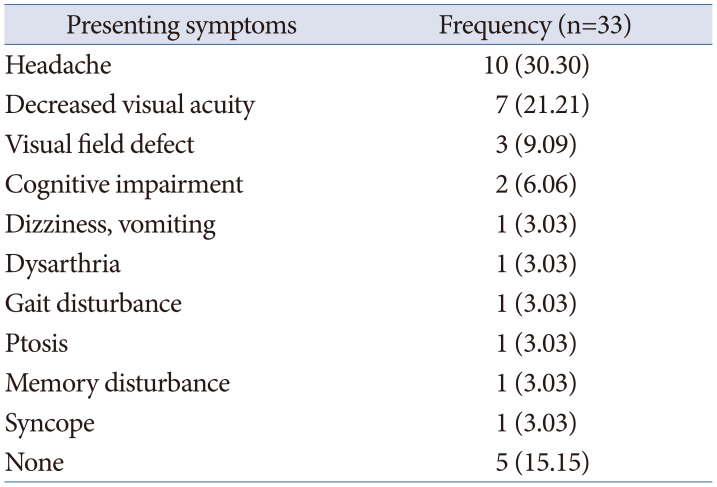
Table 4
Postoperative outcome




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download