Abstract
Acknowledgments
Notes
Ethics Statement: This report was conducted according to the guidelines of the Declaration of Helsinki for biomedical research, and the Institutional Review Board of National Cancer Center exempted the requirement for written informed consent to publish the retrospective case report with a minimal risk for the patient.
Author Contributions:
Conceptualization: Nayoung Han, Ho-Shin Gwak.
Data curation: Yoontae Hong.
Formal analysis: Yoontae Hong, Ho-Shin Gwak.
Methodology: Yoontae Hong, Ho-Shin Gwak.
Project administration: Nayoung Han, Ho-Shin Gwak.
Resources: Ho-Shin Gwak.
Supervision: Ho-Shin Gwak.
Validation: Nayoung Han, Ho-Shin Gwak.
Visualization: Yoontae Hong.
Writing—original draft: Yoontae Hong.
Writing—review & editing: all authors.
Availability of Data and Material
References
Fig. 1
Initial CT, MRI, and histopathologic findings of the right frontal lesion at the first operation in 2013. Axial view of preoperative non-contrast CT (A) and T1-weighted gadolinium-enhanced MRI (B) shows a lobulated, well-circumscribed right frontal mass with homogeneous contrast enhancement. The histopathology slide (C) shows typical meningothelial whorls with increased cellularity, and tumor cells are composed in a syncytial pattern with eosinophilic cytoplasm and indistinct cell borders suggestive of WHO grade 1 meningothelial meningioma (hematoxylin and eosin, ×100).
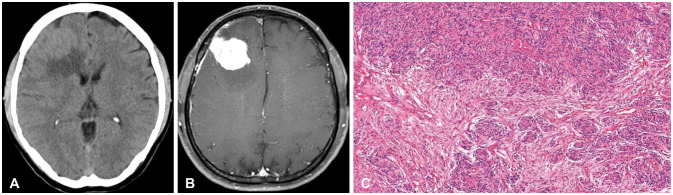
Fig. 2
Follow-up MRI showing new lesions treated by radiotherapies (RTs) in 2015 and 2017. A: Two years after the initial operation, a newly appeared middle frontal convexity lesion was treated by fractionated stereotactic RT of 3,000 cGy/3 fractions. B: One and a half years after the RT, follow-up MRI showed a newly appeared right temporal dural-based mass, and stereotactic radiosurgery with a 1,800 cGy marginal dose was delivered.
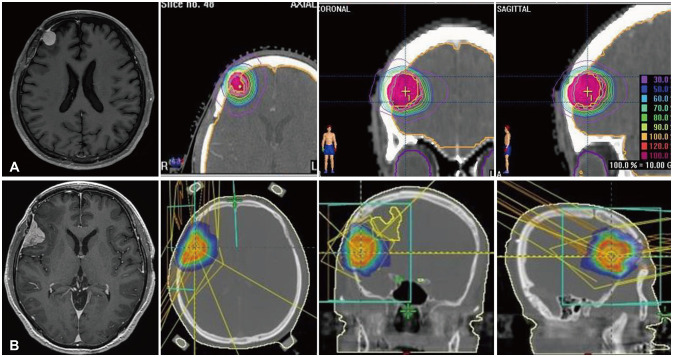
Fig. 3
MRI and histopathologic findings of recurrent lesions at the second operation in 2019. Axial views of preoperative T1-weighted gadolinium-enhanced MRI lesions at the right high frontal (A), middle frontal (B), Sylvian (C), and temporal convexity (D) areas. On the histopathology slides, the previously irradiated (arrows in B and C) middle frontal (F) and Sylvian (G) lesions show treatment-related changes of marked hyalinization with low cellularity. Microscopic findings of the newly appeared middle frontal (E) and temporal convexity (H) lesions (especially the latter) show increased mitoses and prominent nucleoli, compatible with WHO grade 2 atypical meningioma (hematoxylin and eosin, ×100).
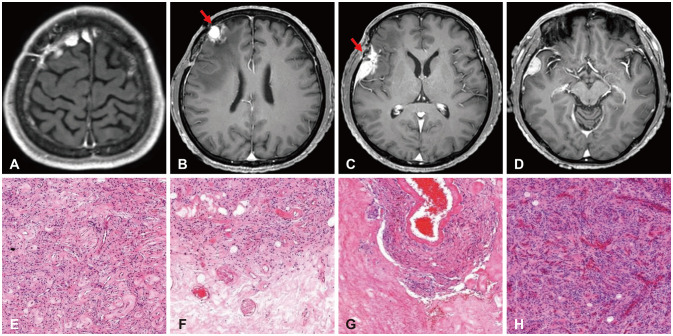
Fig. 4
MRI and histopathologic findings of recurrent lesions at the fifth operation in 2023. Axial views of the preoperative T1-weighted gadolinium-enhanced MRI reveal lesions at the right frontal convexity (red arrow) (A), temporal convexity with necrotic changes (previously irradiated; red arrow) (B), and temporal base with cystic changes (C). Histopathologic examinations show (D) a right frontal lesion (×100), (E) a temporal base lesion with patternless growth (×100), and (F) prominent nucleoli and increased mitoses (×200) suggestive of WHO grade 3 anaplastic meningioma (hematoxylin and eosin).
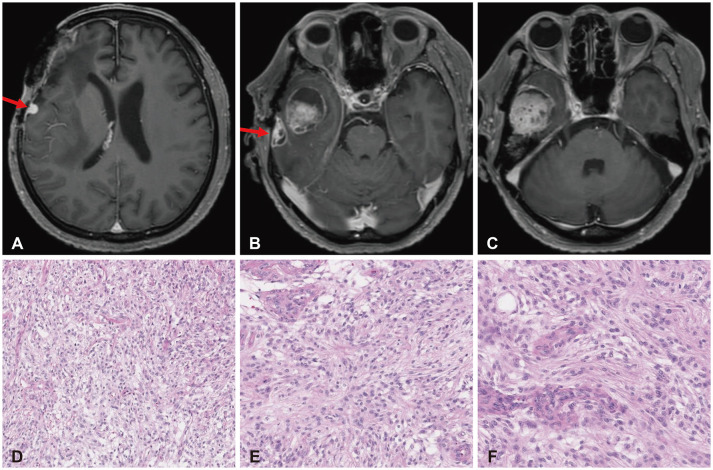
Table 1
Next generation sequencing (NGS Pan cancer panel version 3 for 525 genes) results for first, second, and fifth operation formalin-fixed paraffin-embedded specimen for resection of meningiomas

Note all specimens were identified of Telomerase Reverse Transcriptase Promoter (TERTp) mutation (c.-124C>T), classified as “likely pathogenic” according to ClinVar. No other tumor-related genes were detected. *No copy number variations were reported; †Variant classified “Likely pathogenic” on ClinVar; ‡Allele frequencies for the detected mutation. ND, not detected
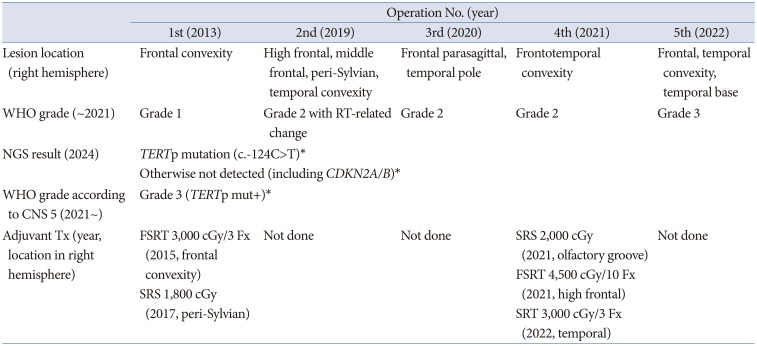
Table 2
Timeline for the case of recurrent meningioma according to operation, lesion location, WHO grade and its NGS result




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download