1Division of Cardiology, Department of Internal Medicine, Wonju Severance Christian Hospital, Yonsei University Wonju College of Medicine, Wonju, Korea.
2Division of Cardiology, Department of Internal Medicine, The Second Affiliated Hospital Zhejiang University School of Medicine, Hangzhou, China.
3Division of Cardiology, Department of Internal Medicine, Samsung Medical Center, Seoul, Korea.
4Division of Cardiology, Department of Internal Medicine, Keimyung University Dongsan Medical Center, Daegu, Korea.
5Division of Cardiology, Department of Internal Medicine, Inje University Ilsan Paik Hospital, Goyang, Korea.
6Division of Cardiology, Department of Internal Medicine, Kangwon National University Hospital, Chuncheon, Korea.
7Division of Cardiology, Department of Internal Medicine, Kyung Hee University Hospital, Seoul, Korea.
8Division of Cardiology, Department of Internal Medicine, Affiliated Hangzhou First People’s Hospital, Zhejiang University School of Medicine, Hangzhou, China.
9Division of Cardiology, Department of Internal Medicine, Hangzhou Normal University Affiliated Hospital, Hangzhou, China.
10Division of Cardiology, Department of Internal Medicine, The 1st Affiliated Hospital of Wenzhou Medical University, Wenzhou, China.
11Division of Cardiology, Department of Internal Medicine, The 2nd Affiliated Hospital of Wenzhou Medical University, Wenzhou, China.
12Division of Cardiology, Department of Internal Medicine, Zhejiang Hospital, Hangzhou, China.
13Division of Cardiology, Department of Internal Medicine, The Third Clinical Institute Affiliated To Wenzhou Medical University, Wenzhou, China.
14Division of Cardiology, Department of Internal Medicine, Ningbo First Hospital, Ningbo, China.
15Division of Cardiology, Department of Internal Medicine, The Affiliated Hospital of Medical School of Ningbo University, Ningbo, China.
16Division of Cardiology, Department of Internal Medicine, Ajou University Hospital, Suwon, Korea.
17Division of Cardiology, Department of Internal Medicine, Yeungnam University Medical Center, Daegu, Korea.
18Division of Cardiology, Department of Internal Medicine, Uijeongbu Eulji Medical Center, Uijeongbu, Korea.
19Division of Cardiology, Department of Internal Medicine, Ulsan University Hospital, University of Ulsan College of Medicine, Ulsan, Korea.
20Division of Cardiology, Department of Internal Medicine, Seoul National University Hospital, Seoul, Korea.

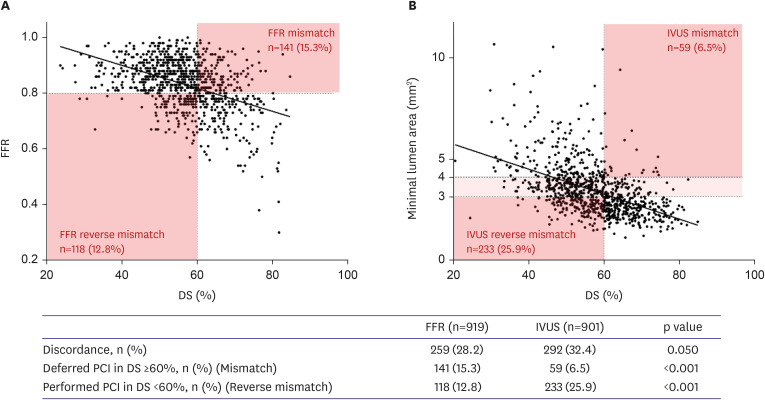
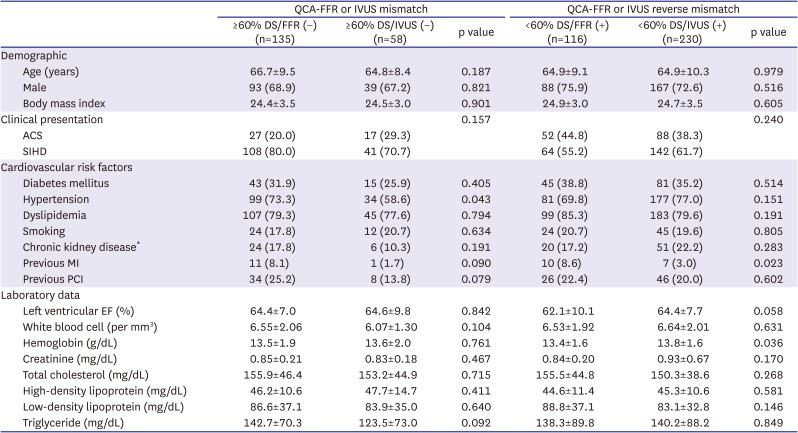
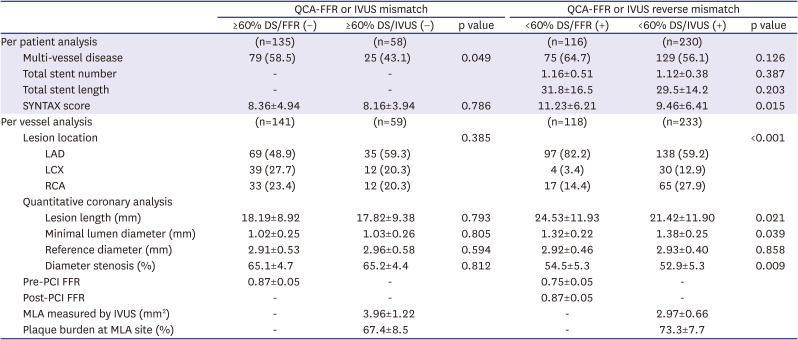
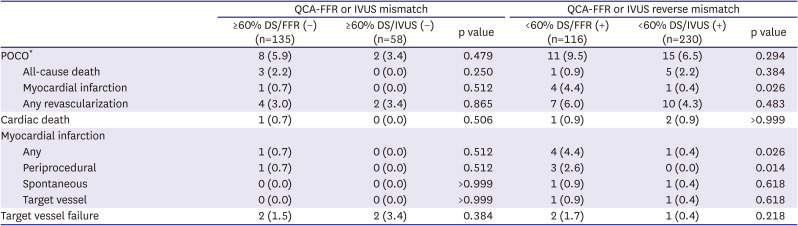




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download