INTRODUCTION
MATERIALS AND METHODS
Research question
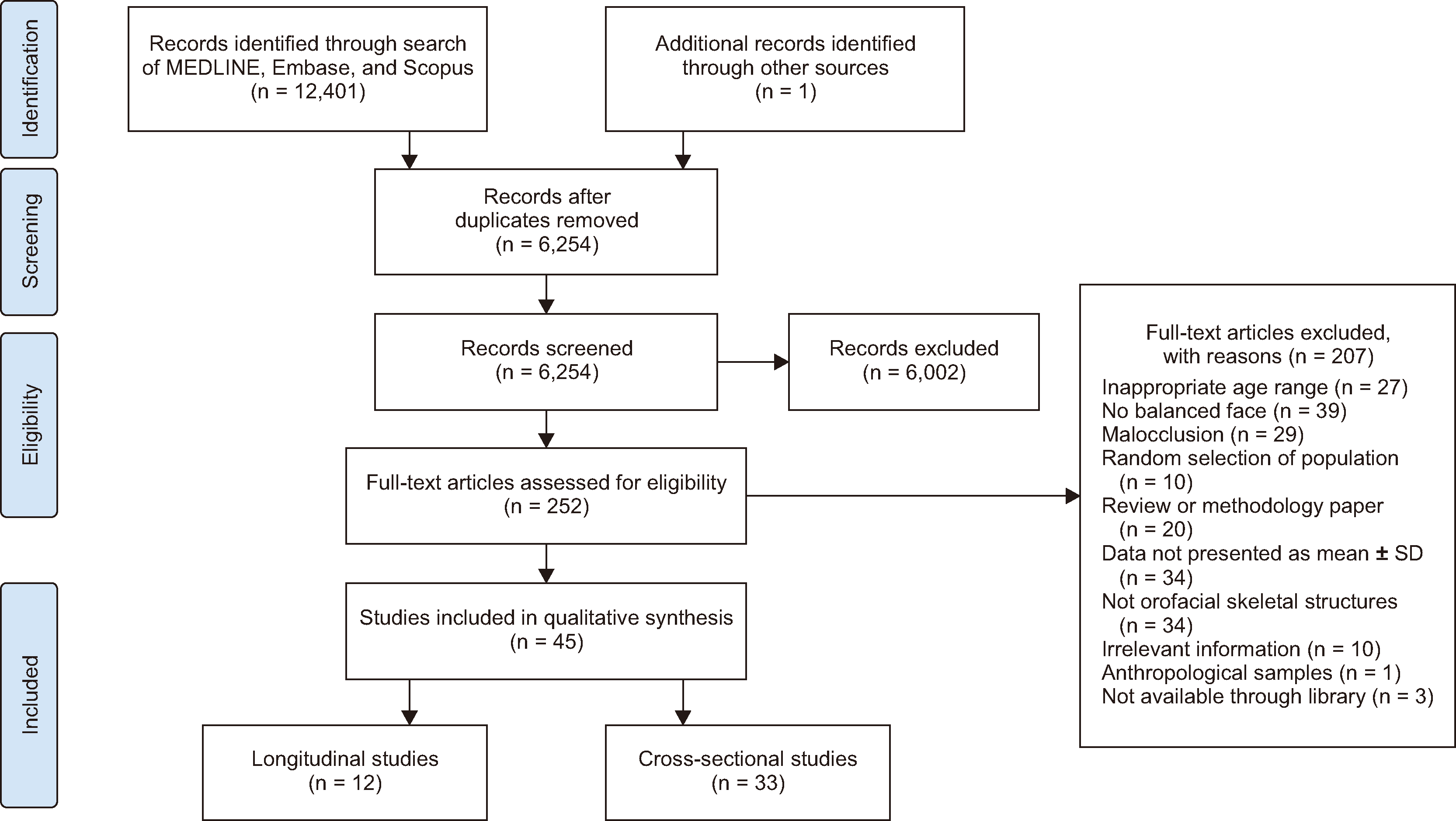
Search methods
Selection criteria
Data collection and analysis
RESULTS
Table 1
| Study | Nationality, sex, sample size (age at measurement timepoint) | Orthodontic measurement | Result |
|---|---|---|---|
| Jamison et al. (1982)43 |
White American: M: n = 20, F: n = 15
Biannually from: 8–12 yr
Annually from: 12–17 yr
|
A-Ptm (Ptm- Jamison)* SNA | A-Ptm significantly increased from 8–17 yr; change in M > in F.SNA increased significantly M, insignificantly in F. |
| ANB, NAPg | ANB, NAPg insignificantly decreased in either sex from 8–17 yr. | ||
| Bishara et al. (1984)1 |
White American: M: n = 20, F: n = 15
Biannually from: 4.5–12 yr
Annually from: 12–17 yr Final set at 25.5 yr GP I: 5–10 yr GP II: 10–15 yr GP III: 15–25.5 yr
Measures at 6, 9, 12, 14, 16, 18 yr
|
A-PNS SNA |
A-PNS: changes in GP II≈ GP I > GP III for M, changes in GP I > GP II > GP III for F. SNA: greatest increase by 1.4° occurred in M in GP II. |
|
Ar-Pg SNB, SNPg |
Ar-Pg: changes in GP II≈ GP I > GP III for M; changes in GP I > GP II > GP III for F. SNB, SNPg: changes in GP I≈ GP II≈ GP III for M; the least amount of increase occurred in GP III in F. |
||
| ANB, NAPg | ANB, NAPg: decreased mostly in GP I, GP III for M; and decreased in GP I, GP II for F. | ||
|
N-ANS’, N-Me, N-ANS’/N-Me,† Ar-Go, S-Go Ar-Go/S-Go S-Go/N-Me SN/MP NSGn |
N-ANS’, N-Me increased the most in GP I, the least in GP III in both sexes. N-ANS’/N-Me increased mostly in GP I in both sexes. Ar-Go, S-Go: changes in GP I≈ GP II≈ GP III for M; but in F, the greatest increase was in GP I. Ar-Go/S-Go decreased in GP I and increased in GP II, GP III in both sexes. S-Go/N-Me, SN/MP changed the most in M, and the least in F during GP III. NSGn: changes in GP I≈ GP II≈ GP III. |
||
| Ursi et al. (1993)2 |
White American: M: n = 16, F: n = 16
Measures at 6, 9, 12, 14, 16, 18 yr
|
S-NS-Ba, NSBa |
S-N: M > F at all ages, especially at 16, 18 yr (P < 0.001). S-Ba: M > F at 16, 18 yr; NSBa: M≈ F. |
|
SNA, A-Nperp Co-A |
SNA, A-Nperp: M≈ F. Co-A: M > F at 9, 14, 16, 18 yr; especially at 16, 18 yr (P < 0.001). |
||
| SNB, Pg-Nperp, Co-Gn | SNB, Pg-Nperp: M≈ F (except at 14 yr, M < F in SNB); Co-Gn: M > F at 16, 18 yr. | ||
|
N-ANS, ANS-Me FH/MP (Go-Me), NBa/PtmGn |
N-ANS: M > F at 14, 16, 18 yr; ANS-Me: M > F at 16, 18 yr. FH/MP: M≈ F; NBa/PtmGn: M < F at 14 yr. |
||
| el-Batouti et al. (1994)41 |
Norwegian: M: n = 35, F: n = 39
Measures at 6, 9, 12, 15, 18 yr
|
NSBa | NSBa: M≈ F from 6–18 yr. |
| SNA | SNA: M > F at 9, 12, 15, 18; M≈ F at 6 yr. SNA increased more in M than F from 6–18 yr; the greatest increase occurred between 9–15 yr. | ||
| SNB | SNB: M > F at 18; M≈ F at 6, 9, 12, 15 yr. SNB increased in both sexes from 6–18 yr (increase in M > in F). | ||
| ANB, NAPg | ANB, NAPg: M > F at 15; M≈ F at 6, 9, 12, 18 yr. | ||
| N-ANS ⊥FH, ANS-Me ⊥FH, N-ANS/ANS-Me (⊥FH‡), S-Go ⊥FH‡, SN/FH, SN/PP,SN/MP (Go-Me) |
N-ANS ⊥FH: M > F at 18; M≈ F at 6, 9, 12, 15 yr. N-ANS/ANS-Me (⊥FH): M < F at 6, 9, 12; M≈ F at 15, 18 yr. ANS-Me ⊥FH, S-Go ⊥FH: M > F at all ages. N-Me ⊥FH, S-Go ⊥FH: increase in M > in F. SN/FH: M≈ F, SN/PP: M < F at all ages. SN/MP: M≈ F at 6, 9, 12, 15 yr; M < F at 18 yr; SN/MP decreased from 6–18 yr in both sexes. |
||
| el-Batouti et al. (1995)61 |
Norwegian: M: n = 35, F: n = 39
White American: M: n = 20, F: n = 15
Measures at 6, 9, 12, 15, 18 yr
|
SNA, SNB, SNPg, FH/NPg, ANB, NAPg, Wits, N-ANS’, N-Me, N-ANS’/N-Me,† Ar’-Go, S-Go, Ar’-Go/S-Go,§ S-Go/N-Me, SN/MP, FH/MP, NSGn, FH/SGn | Norwegian had larger SNA, SNB, SNPg, FH/NPg, Ar’-Go/S-Go; and smaller S-Go, S-Go/N-Me, FH/SGn, NSGn (only in F for NSGn) than white American. |
| Thilander et al. (2005)39 |
Swedish:
Group Umeå: M: n = 55, F: n = 67; measures in 3 age groups at 1) 7 and 10 yr, 2) 10 and 13 yr, 3) 13, 16, 19 and 31 yr
Group Enköping: M: n = 20, F: n = 27; measures at 5, 7, 10 and 13 yr
|
S-N
NSAr, NSBa
|
S-N increased with age; an increase of 1–1.5 mm was even observed from 16–19 yr. In M, one-third of the total increase was noted between 13–16 yr.
NSAr was stable; NSBa decreased around 4°.
|
| SNA | SNA remained constant. | ||
|
Ar-Pg, Goi-Me∥ SNB, SNPg |
Ar-Pg, Goi-Me increased until the young adult period and increase in M > in F. A growth acceleration was noticed between 13–16 yr in M. SNB, SNPg increased continuously during the observation period. |
||
|
ANB NAPg |
ANB decreased during growth. NAPg changed from slight convexity to straight. |
||
| N-ANS’, N-Me, S-GoiANS’-Me†,∥ANS’-Me/N-MeAr–GoiN-Me/S-GoiSN/PP, SN/MP (Downs), MP/PP |
In M, growth acceleration in N-ANS’, N-Me, S-Goi was noted between 13–16 yr. ANS’-Me increased the most between 13–16 yr for both sexes.
ANS’-Me/N-Me was constant during the follow-up.Ar–Goi increased the most from 13–16 yr in M.N-Me/S-Goi decreased continuously.
Only small variations in SN/PP could be seen in both sexes.A continuous decrease in SN/MP, MP/PP with age in both sexes.
|
||
| Al-Taai et al. (2022)40 |
Swedish (Umeå): measures at T1 (13 yr), T2 (16 yr), T3 (31 yr)
T1: M: n = 11, F: n = 19
T2: M: n = 10, F: n = 19
T3: M: n = 11, F: n = 19
|
S-N, N-Ba, NSAr, NSBa, point N | S-N, N-Ba, NSAr increased significantly from T1-T2, T2-T3; NSBa changed insignificantly. Point N moved forward significantly from T1-T2, T2-T3; and downward from T2-T3. |
| SNA, ANS-PNS, point A | SNA increased significantly from T1-T2.ANS-PNS increased significantly from T1-T2, T2-T3. Point A moved forward significantly from T1-T2 and downward from T1-T2, T2-T3. | ||
| SNB, SNPg, Ar-Pg, Go-Me, Ar-Go, Point B, Pg, Me | SNB, SNPg increased significantly from T1-T2.Ar-Pg, Go-Me, Ar-Go increased significantly from T1-T2, T2-T3.Point B, Pg, Me moved forward significantly from T1-T2 and downward from T1-T2, T2-T3. | ||
| ANB | ANB increased significantly from T1-T2, T2-T3. | ||
| N-Me, ANS”-Me,¶ S-Go, PNS”-Go,¶ SN/MP (Go-Me), SN/PP, PP/MP, ArGoMe | N-Me, ANS”-Me, S-Go, PNS”-Go increased significantly from T1-T2, T2-T3.SN/MP, PP/MP decreased significantly from T1-T2. SN/PP increased significantly T1-T2.ArGoMe decreased significantly from T1-T2, T2-T3. | ||
| Stahl de Castrillon et al. (2013)30 |
German: M: n = 16, F: n = 16
Yearly measures from 6–17 yr (except at the age of 14)
|
NSBa, NSArS-N, S-Ba, S-Ar |
NSBa, NSAr: M≈ F, remained constant in both sexes. The length of cranial base (S-N, S-Ba, S-Ar) increased in both sexes. S-N: M > F at 6, 16, 17; S-Ba: M > F at 6 yr. |
| SNA, A-NperpCo-A, ANS-PNS | SNA, A-Nperp: increased with age in M, remained constant in F. Co-A: M > F at 6, 16, 17; ANS-PNS: M > F at 16 yr. | ||
|
SNB, SNPg, Pg-Nperp Co-Gn, Ar-Gn, Go-Me, Co-Go |
SNB, SNPg, Pg-Nperp became larger with age in both sexes.Mandibular length increased with age. Co-Gn: M > F at 6, 7, 15, 16, 17 yr; Ar-Gn: M > F at 6, 15, 16, 17 yr; Go-Me: M > F at 15, 17 yr; Co-Go: M > F at 17 yr. |
||
| ANB, Wits | ANB, Wits: M≈ F at all ages. ANB became smaller with age; Wits value remained constant in both sexes. | ||
| SN/PP, SN/MP (Downs), PP/MP, ArGoMeN-Me, ANS-Me, S-Go |
SN/PP remained constant; SN/MP, MP/PP and ArGoMe became smaller in both sexes: counter-clockwise rotation of mandible with age. N-Me: M > F at 15, 16, 17; ANS-Me: M > F at 15, 17; S-Go: M > F at 17 yr. |
||
| Alió-Sanz et al. (2011)45 |
Spanish: M: n = 22, F: n = 16
Sample divided into 3 age groups GI: 8–11 yr GII: 12–14 yr GIII: 15–18 yr
Annual measures for 6 yr
|
Co-A ANS-PNS SNA Point A |
Co-A increased progressively, means at GIII > GII > GI; the biggest differences were found between GI and GII; increase in M > in F. ANS-PNS: means at GI< GII≈ GIII; increased the most in the GI group. Co-A, ANS-PNS: M > F. SNA increased insignificantly from GI to GIII. The advance of the point A is greater in F than M. |
|
Point ANS Point PNS
N-ANS, SN/PP
|
Point ANS: moved downward in GI≈ GII≈ GIII. Point PNS: moved downward in GIII < GII, GI. From GI to GII, PNS move downward more than ANS. N-ANS: mean at GI< GII≈ GIII; the greatest vertical growth of the maxilla was noted in the GI group; SN/PP: M < F. |
||
| Hamamci et al. (2006)44 |
Turkish: M: n= 22, F: n= 16
Measures at 9, 14, 18 yr
|
SNA A-Nperp Co-A |
SNA significantly increased from 9–14 in F, from 14–18 yr in M. A-Nperp significantly increased in F from 9–14 and 14–18 yr, in M from 9–14 yr. Co-A significantly increased in both sexes between 9 and 14, 14 and 18 yr. |
|
SNB Pg-Nperp Co-Gn |
SNB, Pg-Nperp, Co-Gn significantly increased in both sexes from 9–14, 14–18 yr. Pg-Nperp increased by 6.03 mm for F and 4.71 mm for M from 9–18 yr. Co-Gn increased by 18.7 mm in F and 19.9 mm in M from 9–18 yr. |
||
|
ANB Mx-Md diff |
ANB decreased significantly from 9–14 in F, 14–18 yr in M. Mx-Md diff increased significantly from 9–14, 14–18 yr in M, F. |
||
|
ANS-Me FH/MP (Go-Me) NBa/PtmGn |
ANS-Me increased significantly from 9–14, 14–18 yr in both sexes.FH/MP decreased significantly from 9–14 yr in F, from 9–14 and 14–18 yr in M. NBa/PtmGn changed insignificantly in both sexes. |
||
| Jiménez et al. (2020)28 |
Colombian mestizo (white, African, Amerindian)
Baseline: M: n = 19, F: n = 30
Follow-up: M: n = 10, F: n = 23
Annually from 6–24 yr
|
S-N |
S-N: M≈ F from 6–14 yr, M> F from 16–24 yr. S-N: F had a constant acceleration from 8–16 yr, after that growth rate slowed down. M had a significant acceleration of growth between 14–16 yr, which decreased after 20 yr. |
| Co-A, Co-Gn |
Co-A, Co-Gn: M≈ F from 6–14 yr, M> F from 16–24 yr. Growth of Co-A, Co-Gn plateaued from 8–14 yr in both sexes; M had a pubertal peak between 14–16 yr, while growth in F decreased after 14 yr. |
||
|
N-Me
ANS-Me
S-Goi∥
SN/MP (Downs)
|
N-Me: M > F from 16–24 yr; F had a constant acceleration from 8–14 yr, after that growth rate slowed down; significant pubertal spurt occurred between 14–16 yr in M.
ANS-Me: M > F from 16–24 yr; the growth spurt was between 12–14 in F, and 14–16 yr in M.
S-Goi: M > F from 16–24 yr; the growth spurt was between 10–12 in F, and 14–16 yr in M.
SN/MP decreased with age, no difference between sexes.
|
||
| Chuang (2000)27 |
Taiwanese: M: n = 24, F: n = 24
Biennially at 8, 10, 12 yr
|
S-N, S-Ba, N-Ba, S-Ar, NSBa | S-N, S-Ba, N-Ba, S-Ar increased significantly from 8–10, 10–12 yr in both sexes. S-N, S-Ba, N-Ba: M≈ F; S-Ar: M > F at 8, 12 yr. NSBa: M≈ F; remained constant from 8–12 yr. |
| SNA, ANS-PNS |
SNA, ANS-PNS: M≈ F at 8, 10, 12 yr; SNA insignificantly changed in both sexes.
ANS-PNS increased significantly from 10–12 yr in both sexes.
|
||
| SNB, SNMe, SNGnGo-Gn, Ar-Gn, Ar-Go |
SNB, SNMe, SNGn: M < F; increased insignificantly from 8–12 yr in both sexes. Go-Gn, Ar-Gn increased significantly from 8–10, 10–12 yr; Go-Gn: M≈ F; Ar-Gn: M < F at 12 yr. Ar-Go: M≈ F; increased significantly from 10–12 yr. |
||
| ANB, NAPg | ANB, NAPg: decreased significantly from 8–12 yr; M< F at 12 yr. | ||
| SN/MP (Go-Me), FH/MP, PP/MP, ArGoMe | SN/MP, FH/MP, PP/MP, ArGoMe: decreased insignificantly from 8–12 yr, M≈ F. |
M, male; F, female; ≈, no significant difference; Mx-Md diff, maxillomandibular difference; GP, growth period; NAPg, facial convexity.
*Point Ptm in measurement of maxillary length (A-Ptm), described in Jamison’s study.43
∥Point Goi, gonial intersection, intersection of mandibular plane (Downs) and tangent of mandibular ramus.
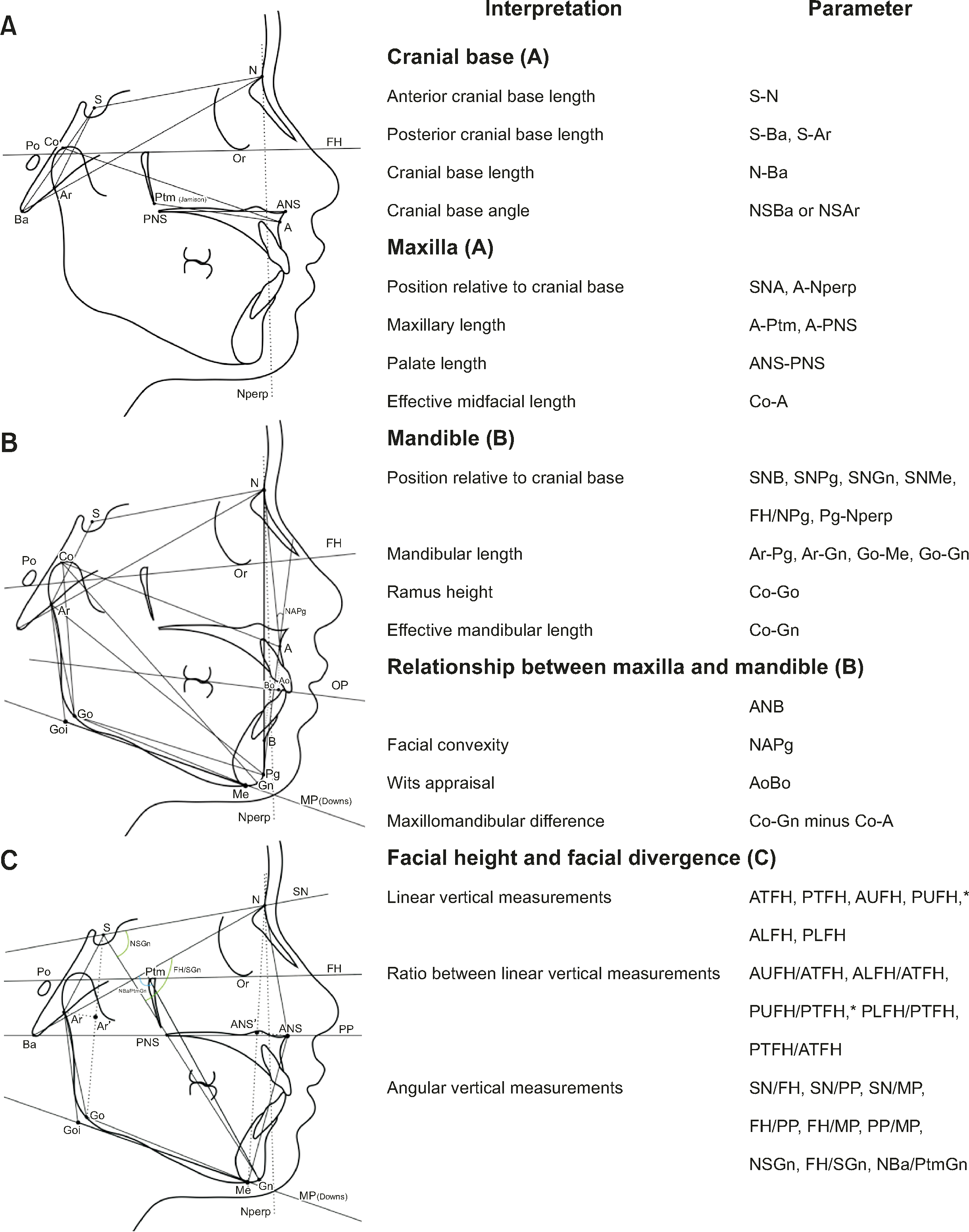
See Figure 2 for definition of each landmark or measurement.
Table 2
| Study | Nationality, sex, sample size, and age | Orthodontic measurement | Result |
|---|---|---|---|
| Bishara and Fernandez (1985)51 |
North Mexican: M: n = 36, 12.76 yr (11–14.16 yr), F: n = 45, 13 yr (11.08–14 yr)
Iowan: M: n = 20, F: n= 15, 12–14 yr
|
SNA, SNB, SNPg, FH/NPg, ANB, NAPg, Wits, N-ANS’, N-Me, N-ANS’/N-Me,* Ar-Go, S-Go, Ar-Go/S-Go, S-Go/N-Me; SN/MP, FH/MP, FH/SGn, NSGn | Significantly larger N-ANS’, N-Me, S-Go in North Mexican M than F;Significantly larger N-ANS’, N-Me, Ar-Go, S-Go in Iowan M than F. |
| Bishara et al. (1990)34 |
Egyptian: M: n = 39, F: n = 51, 12.5 ± 0.6 yr
Iowan: M: n = 33, 13 ± 0.9 yr, F: n = 22, 13 ± 0.8 yr
|
NSAr, NSBa, N-Ba, S-Ba, S-N, SNA, SNB, SNPg, FH/NPg, ANB, NAPg, Wits, N-ANS’, N-Me, N-ANS’/N-Me, Ar’-Go, S-Go, Ar’-Go/S-Go,*,† S-Go/N-Me; SN/MP, FH/MP, FH/SGn, NSGn | Greater N-Ba, S-Ba, S-N and N-ANS’, Ar’-Go, S-Go in Iowan M than F;Larger N-Ba, S-N and smaller NSGn in Egyptian M than F. |
| El-Batran et al. (2008)36 | Egyptian: M: n = 61, F: n = 34, 7.5–9.5 yr (mean, 8.5 yr) | NSBa, N-Ba, S-Ba, S-N, SNA, ANS-PNS, SNB, Go-Me, ANB, NAPg, N-Gn, N-ANS, ANS-Gn; SN/FH, SN/PP, SN/MP (Downs), ArGoiMe‡ | Larger ANS-Gn, SN/MP, and ArGoiMe in Egyptian M than F. |
| Thilander et al. (1982)42 | Swedish: M: n = 27, F: n = 36, 10 yr 9 mo | NSAr, NSBa, S-N, S-Ar, SNA, ANS-PNS, SNB, SNPg, Ar-Goi, Goi-Pg,‡ FH/NPg, ANB, NAPg, N-ANS, N-Me; SN/FH, SN/PP, SN/MP (Downs), FH/PP, FH/MP, PP/MP, ArGoiMe‡ | Smaller S-N, ANS-PNS in 10 yr Norwegian than Swedish. |
| Humerfelt (1970)32 |
Norwegian: M: n = 36, 10 yr 9 mo (10 yr–11 yr 11 mo), F: n = 20, 10 yr 8 mo (10 yr–11 yr 5 mo)
|
NSAr, NSBa, S-N, S-Ar, SNA, ANS-PNS, SNB, SNPg, Ar-Goi, Goi-Pg,‡ ANB, NAPg, N-ANS, N-Me; SN/PP, SN/MP (Downs), PP/MP, ArGoiMe‡ | No significant differences between sexes for angular measurements;Greater linear measurements in M than F. |
| Obloj et al. (2008)33 | Polish: M: n = 39, F: n = 34, 9.25–11.22 yr (10.37 ± 0.52 yr) | NSBa, S-N, SNA, A-Nperp, Co-A, SNB, Pg-Nperp, Co-Gn, ANB, Wits, N-Me, N-ANS, ANS-Me, S-Go, S-Go/N-Me, SN/MP, NBa/PtmGn, ArGoiMe‡ | Greater S-N, N-Me, ANS-Me, S-Go and smaller SNA, A-Nperp, Pg-Nperp, NBa/PtmGn in M than F. |
| Kilic et al. (2010)55 | Turkish: M: n = 33, 13.65 ± 1.47 yr, F: n = 83, 13.42 ± 1.13 yr | A-Nperp, Co-A, Pg-Nperp, Co-Gn, Mx-Md diff, ANS-Me, FH/MP (Go-Me), NBa/PtmGn | Greater Co-A, Co-Gn, ANS-Me in M than F. |
| Hassan (2005)53 | Saudis: M: n = 29, F: n = 33, 9–12 yr | SNA, SNB, FH/NPg, ANB, NAPg, ANS-Me; FH/MP (Downs), SN/MP (Downs), FH/SGn | No significant differences between M and F children;Significantly greater NAPg, smaller ANS-Me in children than Saudis adults. |
| AlShayea et al. (2022)26 |
Saudis: F: n = 140, 11 ± 1 yr (10–13 yr)
|
S-N, S-Ar, NSAr, SNA, A-Nperp, Co-A, SNB, Pg-Nperp, Pg-NB, FH/NPg, Co-Gn, Go-Me, ANB, NAPg, ANS-Me, N-Me, Ar-Go, S-Go, S-Go/N-Me, SN/OP, FH/OP, FH/MP (Go-Me), FH/SGn, NBa/PtmGn, ArGoMe | Greater SNA, SNB, ANB, SN/OP, FH/OP, FH/MP (Go-Me), FH/SGn; NBa/PtmGn; and smaller S-Ar, NSAr, Co-A, Pg-Nperp, Pg-NB, FH/NPg, Co-Gn, Go-Me, ANS-Me, S-Go/N-Me, ArGoMe in Saudis girls than British Caucasian adults. |
| Hamdan and Rock (2001)62 | Jordanian: M: n = 33, F: n = 32, 14–17 yr (15.5 ± 0.5) | SNA, SNB, ANB, PP/MP | Smaller PP/MP in 15.5 yr Jordanian than British adults. |
| Gleis et al. (1990)54 | Israeli: M: n = 18, 12–16.5 yr, F: n = 22, 11–14 yr | SNA, SNB, Pg-NB, FH/NPg, ANB, NAPg, SN/GoGn, FH/MP (Downs), FH/PP, FH/SGn | Greater FH/SGn in M than F. |
| Aleksić et al. (2012)35 | Serbian: M: n = 36, 9 ± 0.17 yr, F: n = 42, 9 ± 0.43 yr | S-N, SNA, SNB, N-Me, S-Goi, S-Goi/N-Me; SN/PP, SN/MP (Downs), ArGoiMe‡ | Larger S-N, N-Me, SN/MP and smaller S-Goi/N-Me, SNA, SNB in M than F. |
| Huang et al. (1998)48 |
White American: M: n = 32, F: n = 35
African American: M: n = 39, F: n = 30
2 groups: young (6–12 yr), old (12–18 yr)
|
SNA, SNB, ANB, Wits | No significant differences between M and F. |
| Alexander and Hitchcock (1978)63 |
Black American: n = 50, 8–13 yr (10.18 ± 1.38 yr)
|
SNA, SNB, SNPg, ANB, SN/MP (Downs), NSGn | Greater SNA, ANB, SN/MP in 10 yr black American than 10 yr white Southern American. |
| Barter et al. (1995)38 |
South-African: M: n = 50, 14 yr 1 mo (11 yr 4 mo–16 yr 1 mo), F: n = 54, 13 yr 6 mo (11 yr 4 mo–16 yr 9 mo)
|
S-N, SNA, Co-A, SNB, FH/NPg, Co-Gn, Go-Gn, ANB, Wits, N-ANS, ANS-Me; SN/PP, SN/GoGn, ArGoGn, NSGn | Smaller SN/PP in M than F. |
| Ajayi (2005)47 |
Nigerian Igbo: M: n = 66, F: n = 34, 11–13 yr (12.6 ± 0.6 yr)
|
SNA, SNB, ANB, FH/MP (Go-Me) | No significant differences between M and F. |
| Folaranmi and Isiekwe (2013)59 | Nigerian: M: n = 40, F: n = 60, 12.2 yr (12–15 yr) | N-ANS, ANS-Me, N-Me, ANS-Me/N-Me | No significant differences between M and F. |
| Beugre et al. (2007)64 |
Ivorian: M: n = 26, F: n = 27, 9.5–17 yr
Senegalese: M: n = 25, F: n = 25, 12–16 yr
Chadian: M: n = 31, F: n = 31, 12–16 yr
|
SNA, SNB, SNPg, ANB, Wits, S-Go, N-Me, S-Go/ N-Me; FH/MP, SN/MP (Go-Gn), SN/PP | No sexual dimorphism in any ethnic group (except greater S-Go, N-Me in Senegalese M than F). |
| Kapila (1989)49 | Kikuyu: M: n = 28, 11.5 yr, F: n = 28, 10.85 yr | SNA, SNB, ANB, FH/MP (Go-Gn) | Smaller SNB in M than F. |
| Sobreira et al. (2011)25 |
Black Brazilian: F: n = 35
White Brazilian: F: n = 35
3 groups: 8 yr (n = 22), 9 yr (n = 18), 10 yr (n = 30)
|
ANS-Me/N-Me, S-Go/N-Me, Ar-Go/S-Go, Ar-Go/ANS-Me | No significant differences among 3 age groups in both races. |
| de Freitas et al. (2010)65 |
White Brazilian: M: n = 25, F: n = 25, 13.17 ± 1.07 yr
Black Brazilian: M: n = 28, F: n = 28, 13.24 ± 0.56 yr
|
SNA, A-Nperp, SNB, Co-Gn, Pg-Nperp, Pg-NB, ANB, NAPg, Wits, FH/MP (Go-Me), SN/GoGn | Greater SNA, A-Nperp, SNB, Pg-Nperp, ANB, NAPg; and smaller Co-Gn, Pg-NB, FH/MP, SN/GoGn in black than white Brazilian. |
| de Freitas et al. (2007)50 |
White Brazilian: M: n = 37, F: n = 37, 13.71 ± 0.84 yr
Black Brazilian: M: n = 28, F: n = 28, 13.86 ± 0.92 yr
|
SNA, SNB, ANB, N-Me, N-ANS’, ANS’-Me, N-ANS’/N-Me, ANS’-Me/N-Me, S-Go, S-Ar’, Ar’-Go, S-Ar’/S-Go, Ar’-Go/S-Go*,† | Significantly greater S-Go, N-ANS’, S-Ar’, S-Ar’/S-Go; and smaller Ar’-Go/S-Go in black M than F;Significantly greater S-Ar’, S-Ar’/S-Go and smaller Ar’-Go/S-Go in white M than F. |
| Janson et al. (2011)52 |
White Brazilian: M: n = 20, F: n = 20, 13.02 yr (11.89–15.03 yr)
Afro-Caucasian Brazilian: M: n = 20, F: n = 20, 13.02 yr (12–14.30 yr)
|
SNA, SNB, ANB, N-Me, N-ANS’, ANS’-Me, S-Go, S-Ar’, Ar’-Go*,† | No significant differences between Caucasian Brazilian M and F;Greater SNB, S-Go, S-Ar’ in Afro-Caucasian Brazilian M than F. |
| Storniolo-Souza et al. (2021)57 |
White Brazilian: M: n = 20, 13.57 ± 1.03 yr, F: n = 20, 13.70 ± 0.87 yr
Japanese: M: n = 16, 15.56 ± 2.51 yr, F: n = 17, 15.65 ± 2.45 yr
Japanese-White-Brazilian: M: n = 15, 14.19 ± 1.01 yr, F: n = 17, 13.22 ± 1.04 yr
|
A-Nperp, Co-A, Pg-Nperp, Co-Gn, Mx-Md diff, ANS-Me, FH/MP (Go-Me), NBa/PtmGn |
Significantly greater Co-A, Co-Gn, Mx-Md diff, and smaller A-Nperp in Japanese M than F;Significantly greater ANS-Me, and smaller Pg-Nperp in Japanese-White-Brazilian M had than F;
No significant differences between White-Brazilian M and F.
|
| Vieira et al. (2014)58 | Japanese-Caucasian-Brazilian: M: n = 15, F: n = 15, 14 yr (11.91–16.61 yr) | N-Me, N-ANS’, ANS’-Me, N-ANS’/N-Me, ANS’-Me/N-Me, S-Go, S-Ar’, Ar’-Go, S-Ar’/S-Go, Ar’-Go/S-Go*,† | Greater N-Me, ANS’-Me, S-Go, S-Ar’ in M than F. |
| Singh Rathore et al. (2012)60 | Mewari: M: n = 50, F: n = 50, 11–13 yr | SNA, SNB, ANB, SN/MP (Go-Gn) | Greater ANB, and smaller SNB in Mewari children than white adults. |
| Anuradha et al. (1991)46 | North Indian: M: n = 30, F: n = 30, 4–5 yr | SNA, SNB, ANB, SN/ MP (Go-Gn) | Insignificant difference between M and F. |
| Singh et al. (2019)56 | Lingayat: M: n = 110, F: n = 110, 11–13 yr | FH/NPg, NAPg, FH/MP (Downs) | Greater NAPg in M than F. |
| Moldez et al. (2006)31 |
Filipino: M: n = 78, F: n = 79
4 groups: GI: 7 yr, GII: 9.5 yr, GIII: 14 yr, GIV: 22 yr
|
S-N, SNA, SNB, Co-Gn, Co-Go, FH/NPg, ANB, NAPg, N-Me, N-ANS, ANS-Me; FH/PP, SN/FH, SN/PP, SN/MP (Go-Me), FH/SGn |
Greater S-N, N-Me, N-ANS, Co-Go, Co-Gn in M than F in GI, GIII, GIV;Greater ANS-Me in M than F in GIII, GIV;
Insignificant differences between sexes for linear measurements in GII;
Insignificant differences between sexes in GI, GII, GIII for angular parameters; larger SNB, SN/FH, and smaller SN/PP, SN/MP in M than F in GIV.
|
| Zhao et al. (2013)37 |
Chinese: M: n = 16, F: n = 16, 12 yr 11 mo–13 yr 1 mo
White: M: n = 16, F: n = 16, 13 yr
|
S-N, S-Ba, NSAr, SNA, ANS-PNS, SNB, SNPg, FH/NPg, Go-Pg, Ar-Go
ANB, NAPg, N-ANS, ANS-Me, N-Me; FH/MP (Go-Gn), SN/MP, PP/MP, FH/SGn
|
Insignificant differences between white M and F;
Greater N-ANS in Chinese M than F.
|
| Gu et al. (2011)66 |
Southern Chinese: M: n = 70, 12.4 ± 0.6 yr, F: n = 60, 12.5 ± 0.4 yr
Northern Chinese: M: n = 50, 12.8 ± 1.8 yr, F: n = 50, 12.4 ± 1.2 yr
British: M: n = 43, F: n = 43, 12 yr
|
A-Nperp, Co-A, Pg-Nperp, Co-Gn, Mx-Md diff, FH/MP (Go-Me), ANS-Me |
The smallest FH/MP in British;
The greatest Co-A, Co-Gn, FH/MP, ANS-Me; and smallest Pg-Nperp in Northern Chinese.
|
| Pan et al. (1996)24 | Chinese: M: n = 25, F: n = 32, 12 yr | A-Nperp, Co-A, Pg-Nperp, Co-Gn, ANS-Me | Greater Co-A, A-Nperp in M than F. |
| Chang et al. (1993)29 | Taiwanese: M: n = 80, F: n = 802 groups: 11 yr 1 mo–12 yr 8 mo (M: n = 40, F: n = 40), young adult (M: n = 40, F: n = 40) | S-N, S-Ar, NSAr, Co-A, Co-Gn, Ar-Goi, Goi-Pg,‡ ATFH, AUFH, ALFH, PTFH, PUFH, PLFH, AUFH/ATFH, ALFH/ATFH, AUFH/ALFH, PUFH/AUFH, PLFH/ALFH, PTFH/ATFH,§ SN/FH, SN/PP, FH/PP, SN/MP (Downs), FH/MP, PP/MP |
No differences between 2 age groups in NSAr, AUFH/ATFH, ALFH/ATFH, AUFH/ALFH, PUFH/AUFH, SN/FH, SN/PP, FH/PP;
Greater S-N, S-Ar, Co-A, Co-Gn, Ar-Goi, Goi-Pg, ATFH, ALFH, AUFH, PTFH, PUFH, PLFH, PLFH/ALFH, PTFH/ATFH (except for PTFH/ATFH in F) in adult than child;
Smaller SN/MP, FH/MP, PP/MP (only in M) in adult than child;
Greater parameters in M than F in child and adult for S-N, S-Ar, Co-Gn, ATFH, ALFH; and only in adult for Co-A, Ar-Goi, Goi-Pg, AUFH, PTFH, PUFH, PLFH;Smaller PLFH/ALFH, PTFH/ATF in M child than F, but greater in adult M;
Greater SN/MP, FH/MP, PP/MP in M child than F, but smaller in adult M.
|
M, male; F, female; ≈, no significant difference; Mx-Md diff, maxillomandibular difference; NAPg, facial convexity; ATFH, anterior total facial height; AUFH, anterior upper facial height; ALFH, anterior lower facial height; PTFH, posterior total facial height; PUFH, posterior upper facial height; PLFH, posterior lower facial height.
‡Point Goi, gonial intersection, intersection of mandibular plane (Downs) and tangent of mandibular ramus.
See Figure 2 for definition of each landmark or measurement.
Cranial base
Figure 2




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download