INTRODUCTION
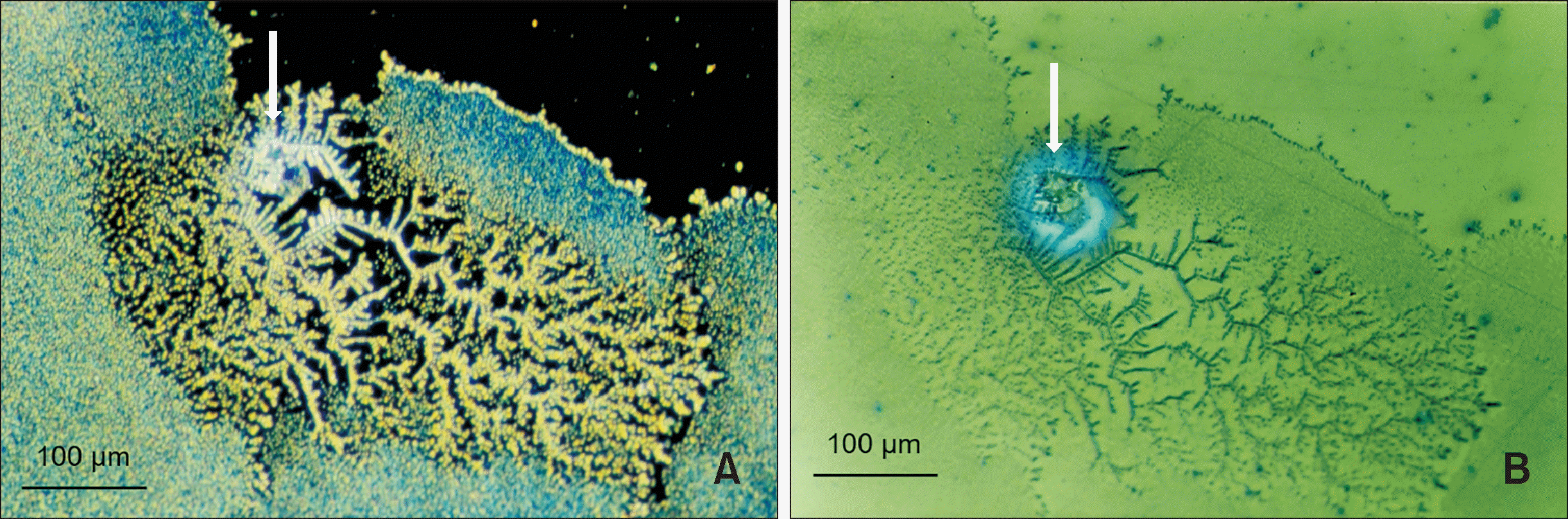
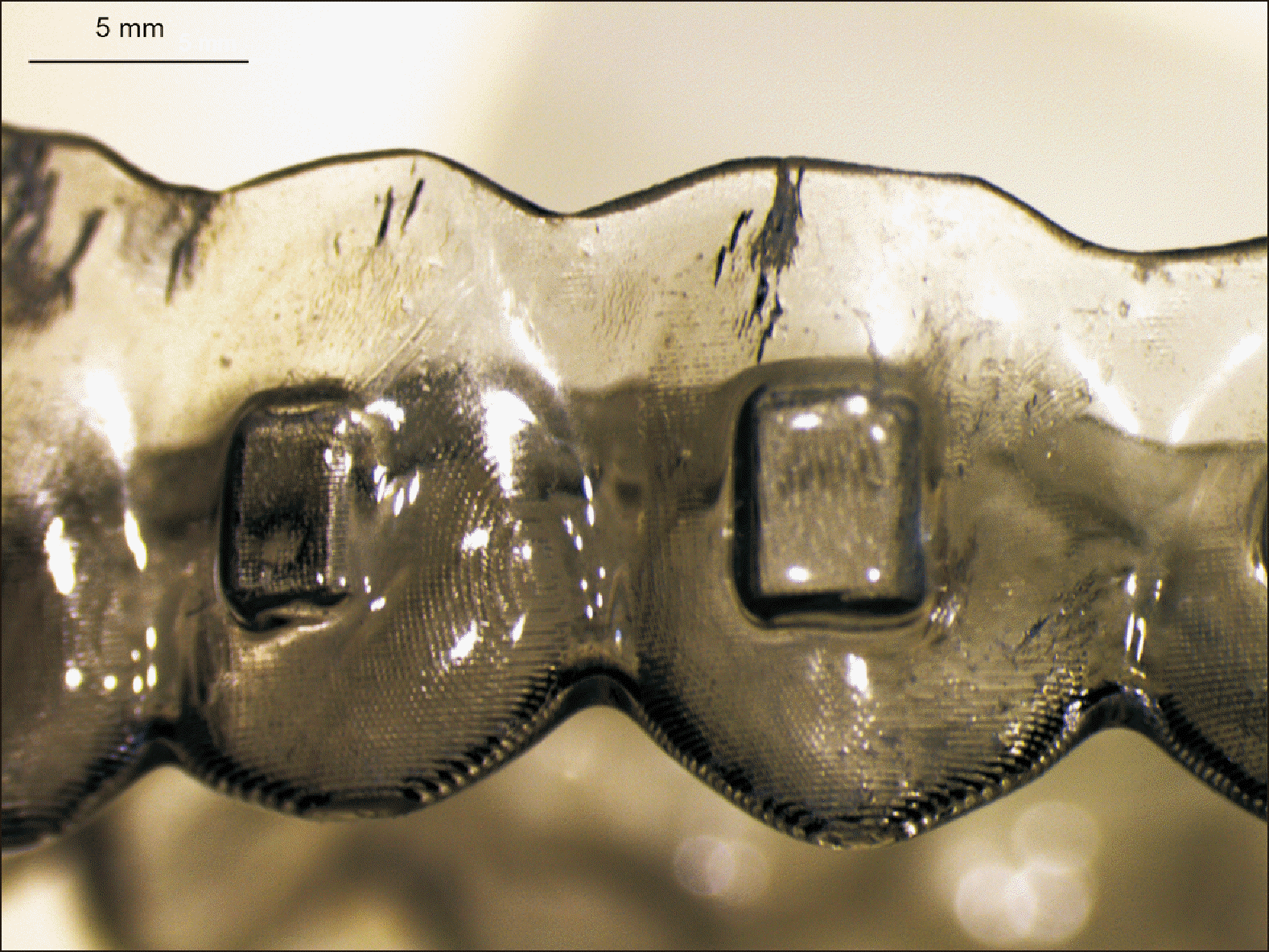
1) examine the ageing pattern of aligners in vivo, under actual clinical scenarios;
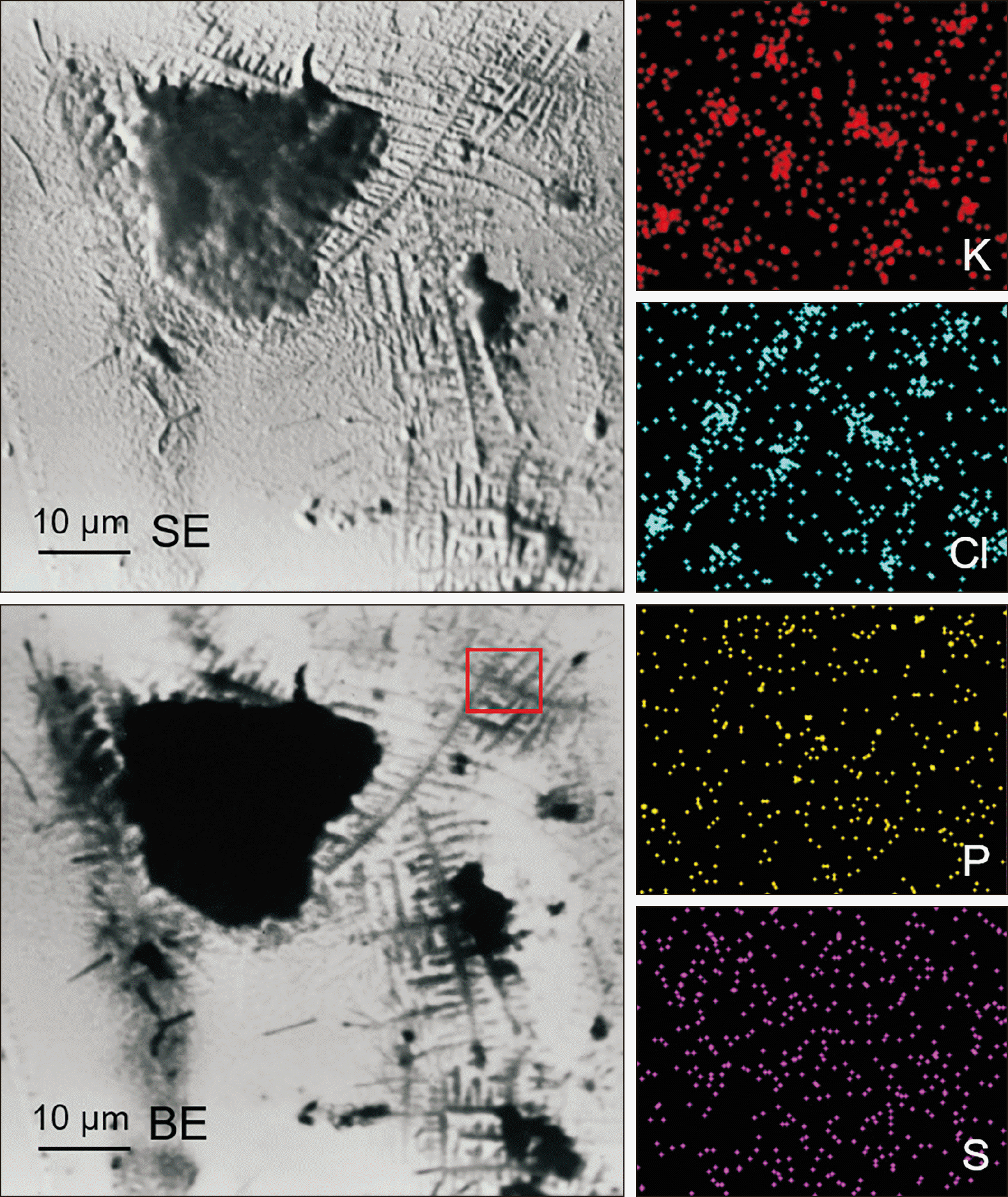
2) describe the alterations of the mechanical properties of the aligners induced by in vivo ageing, and identify a link to the discrepancy between predicted and actual clinical outcome;
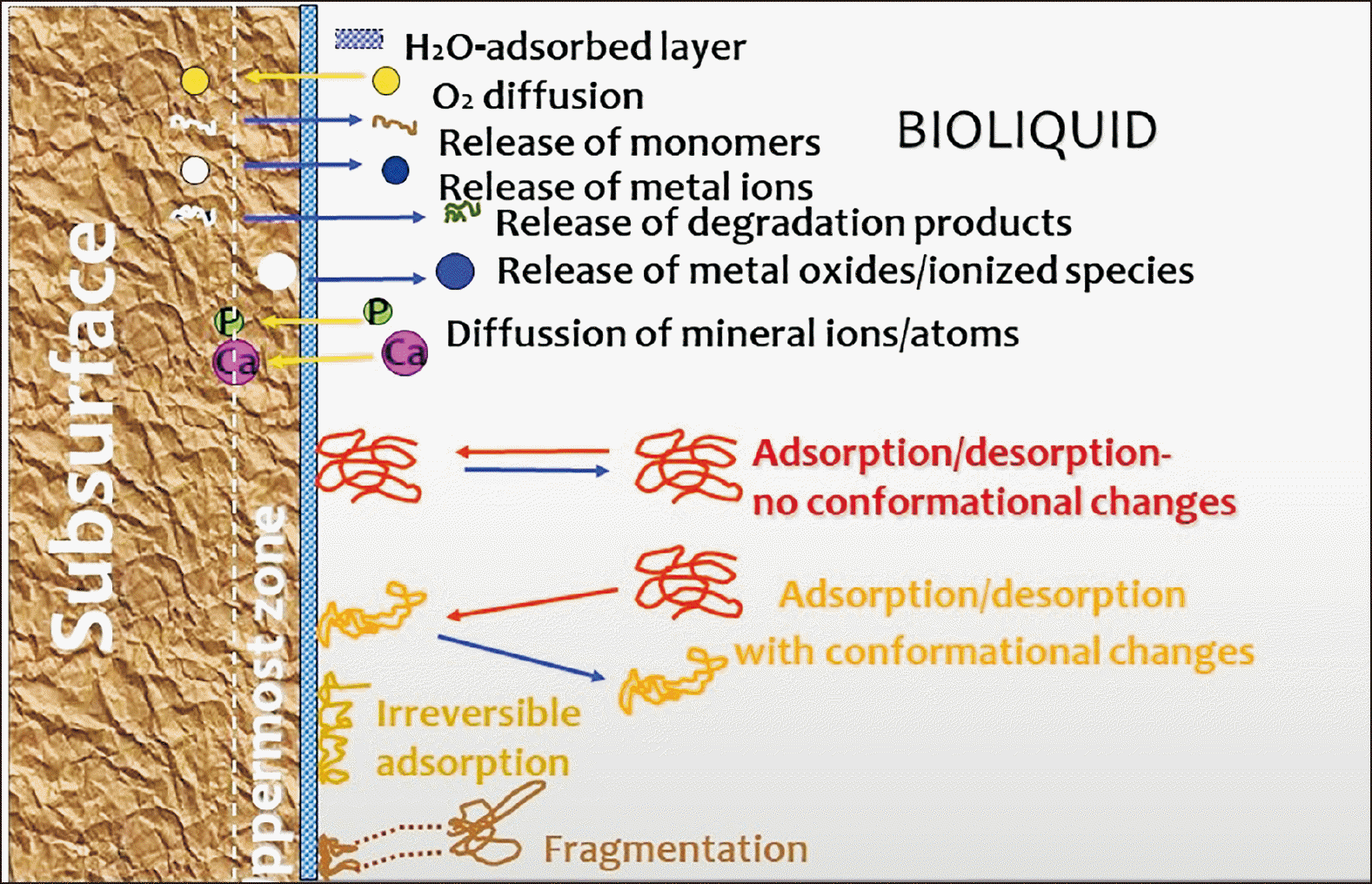
3) summarize the evidence on the composition and kinetics of the eluted compounds in vitro and in vivo; and propose ways to bypass the inefficiency of the system.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download