Dieulafoy’s lesion (DL) is a vascular malformation that causes profound gastrointestinal (GI) bleeding. The incidence, accounting for approximately 1% to 2%, is relatively uncommon.1 Approximately 70% of these lesions are located in the proximal stomach, within 6 cm of the esophagogastric junction. Other common locations include the duodenum and esophagus. DL usually manifests as a small vascular protrusion without significant mucosal defect.2
A 73-year-old Thai man with a history of diabetes mellitus, hypertension, and a minor ischemic stroke presented with hematemesis. He was admitted for the treatment of coronavirus disease 2019 (COVID-19) pneumonia. During his hospitalization, he received oxygen support, intravenous remdesivir, systemic corticosteroids, and enoxaparin dose for 1 week for thrombotic prophylaxis. His overall condition gradually improved within a week without evidence of GI bleeding. In the 2nd week of his admission, he developed hypovolemic shock, evidenced by the passage of copious melena. The patient was resuscitated and finally stabilized after fluid therapy and blood transfusion. He denied administration of over-the-counter drugs and had a history of abdominal surgery. His hemoglobin level decreased from 11.9 to 8 g/dL, whereas his platelet count and coagulogram were within normal limits. His blood urea nitrogen level was 40.3 mg/dL, with a creatinine level of 0.7 mg/dL. Esophagogastroduodenoscopy was performed that did not lead to significant lesions, except for minimal blood-stained mucosa at the second part of the duodenum. Hence, push enteroscopy was performed, which revealed a 2.5 cm, round, subepithelial mass with a large central defect covered by an adherent clot in the proximal jejunum (Fig. 1). The initial impression was gastrointestinal stromal tumor (GIST) with suspicion of ongoing bleeding. Thus, an anatomical landmark was located by applying a hemostatic clip adjacent to the lesion, and then abdominal computed tomographic angiography (CTA) was promptly performed. Radiological examination revealed a round-shaped arterial enhancing lesion in the jejunum without active contrast extravasation. This lesion was persistently enhanced in the portal venous phase, suggesting a vascular lesion or tumor (Fig. 2A). This imaging revealed no evidence of abnormal vessels or lymphadenopathy. An interventional radiologist was consulted due to the continued passage of maroon stool and hemodynamic instability. Angiography was performed with the intention of angioembolization. After super-selection angiography into the jejunal branch of the superior mesenteric artery, an accumulation of contrast media, supplied by multiple small jejunal branches, was identified (Fig. 2B). Embolization was not performed as active contrast extravasation could not be demonstrated. Subsequently, surgery was performed to stop the bleeding. Exploratory laparotomy revealed a non-active bleeding subepithelial mass, excised along with 20 cm of small bowel. The bleeding stopped immediately after the surgery. The lesion revealed a large thrombus within the submucosa (Fig. 3A). The histopathological examination revealed a submucosal persistent-caliber artery that was dilated with a recent thrombus, and the lesion was connected to the overlying mucosal defect (Fig. 3B). The vascular wall of the lesion was exceedingly thickened compared to the normal submucosal artery (Fig. 3C). The overall pathological finding is consistent with DL. The postoperative course of the patient was uneventful, without further bleeding.
DL is a condition characterized by an enlarged blood vessel beneath the GI mucosa that erodes the overlying tissue, resulting in bleeding without ulcers or erosion.3 Some predisposing factors have been identified, including male sex, advanced age, administration of antithrombotic drugs, and comorbidities, such as cardiovascular disease and chronic renal insufficiency despite the lack of knowledge regarding its exact cause.4 One case report recently described a patient who experienced rectal bleeding due to DL while hospitalized for severe COVID-19 pneumonia and respiratory failure.5 This indicates that severe medical conditions, including SARS-CoV-2 infection, may precede this vascular bleeding. The jejunal location is extremely uncommon and predominantly observed in patients aged 70–80 years. The proposed mechanism for this occurrence is related to age-related wear and tear of the submucosal vessels.6 DL typically presents as a small pigmented protuberance, measuring <10 mm, with minimal mucosal defect. It often exhibits active bleeding during endoscopy.2 We demonstrated an atypical appearance of DL, characterized by a large subepithelial mass with a significant central defect. This unique manifestation indicates a tumor rather than a vascular lesion. GIST was the common differential diagnosis for the bleeding subepithelial mass according to the current endoscopic evaluation. Few cases of DL mimicking GIST have been reported in the literature, with an average size of 2 cm in the stomach and 1 cm in jejunum (Table 1).7-9 Other potential rare causes of bleeding subepithelial mass include glomus tumor and arteriovenous malformation.10 Although endoscopic findings can make definitive diagnosis difficult, the management approach is consistent. Direct endoscopic intervention is not the preferred method, as clot removal or biopsy might exacerbate bleeding, posing a life-threatening risk. Patients with subepithelial mass bleeding should undergo CTA followed by embolization, with surgical resection as the definitive treatment. Informed consent was obtained for this case report.
REFERENCES
1. Kim SE, Kim HJ, Koh M, et al. A practical approach for small bowel bleeding. Clin Endosc. 2023; 56:283–289.

2. Baxter M, Aly EH. Dieulafoy's lesion: current trends in diagnosis and management. Ann R Coll Surg Engl. 2010; 92:548–554.
3. Malik TF, Anjum F. Dieulafoys Lesion causing gastrointestinal bleeding. StatPearls;2023.
4. Sakai E, Ohata K, Nakajima A, et al. Diagnosis and therapeutic strategies for small bowel vascular lesions. World J Gastroenterol. 2019; 25:2720–2733.

5. Tahir MM, Saeed S, Hlaing SS, et al. Dieulafoy lesion causing lower GI bleeding: a case of COVID-19 critical illness prompting unusual presentation of a more rare condition. Critical Care. 2023; 162:A926–A927.

6. Malik A, Inayat F, Goraya MH, et al. Jejunal Dieulafoy's lesion: a systematic review of evaluation, diagnosis, and management. J Investig Med High Impact Case Rep. 2021; 9:2324709620987703.

7. Badwal S, Jain M, Rastogi A, et al. Dieulafoy disease of the stomach presenting as mass lesion: a case report. Indian J Pathol Microbiol. 2005; 48:211–213.
8. Jeong HW, Kim JY, Kim SJ, et al. A case of a Jejunal Dieulafoy's lesion mimicking a submucosal tumor. Korean J Gastrointest Endosc. 2008; 37:438–442.
Fig. 1.
Push enteroscopy reveals a 2.5 cm round subepithelial mass with a large central defect covered by an adherent clot in the proximal part of the jejunum.
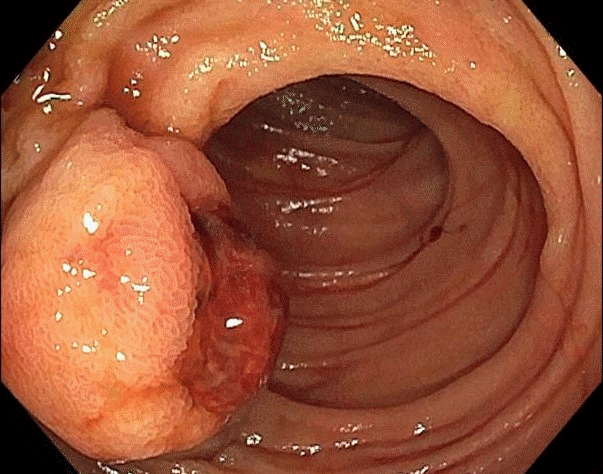
Fig. 2.
(A) Arterial phase computed tomographic angiography shows a round-shaped 2×1.4 cm arterial enhancing lesion in the proximal jejunum (arrow) adjacent to hemostatic clip (*) without active contrast extravasation within bowel loops. (B) The angiography of the jejunal branch of the superior mesenteric artery reveals an accumulation of contrast media at the proximal jejunum (arrow) near the hemostatic clip (*), which is supplied by multiple small jejunal branches. No demonstrable active contrast extravasation is seen in the study.
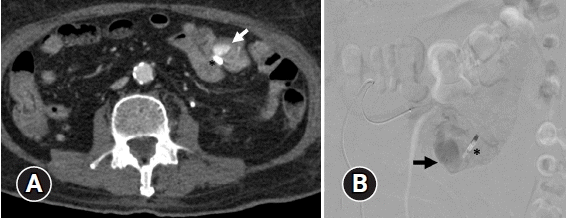
Fig. 3.
(A) The gross lesion reveals a large thrombus within the submucosa. (B) The histopathology reveals a submucosal persistent-caliber artery, dilated with a recent thrombus (*) (hematoxylin and eosin stain, ×10). The overlying mucosal defect (arrow) is connected to the lesion. (C) The vascular wall of the lesion (dotted line) is exceedingly thickened compared to the normal submucosal artery (*) (hematoxylin and eosin stain, ×200).
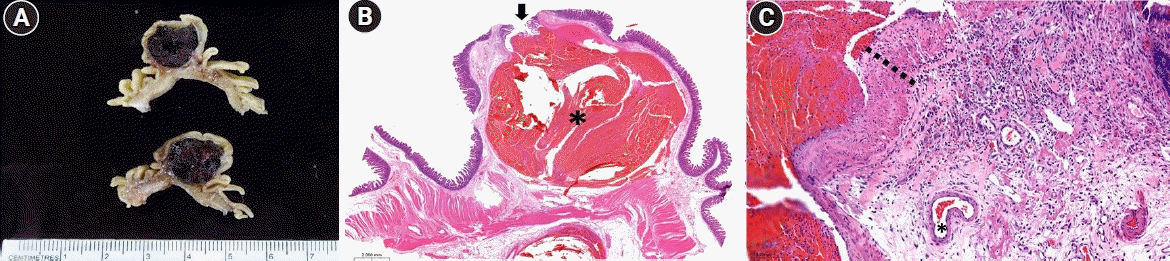
Table 1.
Published case reports of Dieulafoy’s lesion resembling a gastrointestinal stromal tumor
| Study | Sex/age (yr) | Comorbidity | Presentation | Location, diameter | Diagnostic methods | Treatment | Outcome |
|---|---|---|---|---|---|---|---|
| Badwal et al.7 | Female/55 | None | Hematemesis | Fundus of stomach, 2 cm | Esophagogastro-duodenoscopy | Surgical resection | Uneventful |
| Jeong et al.8 | Male/32 | None | Hematochezia | Proximal jejunum, 1.5 cm | Double balloon enteroscopy | Surgical resection | Uneventful |
| Zhao et al.9 | Male/41 | None | Hematochezia | Proximal jejunum, 1 cm | Single balloon enteroscopy | Surgical resection | Uneventful |
| This case | Male/73 | Hypertension, diabetes mellitus, ischemic stroke | Hematemesis | Proximal jejunum, 2.5 cm | Push enteroscopy | Surgical resection | Uneventful |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download