Abstract
Background
Tixagevimab/cilgavimab (Tix/Cil) shows promise as a prophylactic treatment against coronavirus disease 2019 (COVID-19) in solid organ transplant recipients (SOTRs). This study was performed to assess the effectiveness of Tix/Cil for preexposure prophylaxis against COVID-19 in this population.
Methods
We systematically searched the Cochrane Library, Web of Science, PubMed, and Embase databases to identify articles relevant to our study up to December 15, 2023. Comprehensive Meta-Analysis (ver. 3.0) was used for data analysis.
Results
The meta-analysis included seven eligible retrospective studies, encompassing a total of 4,026 SOTRs. The analysis revealed significant differences in SOTRs who received Tix/Cil preexposure prophylaxis relative to those who did not. Specifically, these differences were observed in the incidence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection (odds ratio [OR], 0.30; 95% confidence interval [CI], 0.15–0.60), hospitalization (OR, 0.24; 95% CI, 0.08–0.70), and intensive care unit admission (OR, 0.07; 95% CI, 0.02–0.22). However, mortality rate did not differ significantly between the two groups (P=0.06).
Several studies have indicated that solid organ transplant recipients (SOTRs) infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus responsible for coronavirus disease 2019 (COVID-19), experience higher rates of mortality and hospitalization compared to the general population [1–3]. Despite the effectiveness of COVID-19 vaccines in augmenting the immune responses of SOTRs, approximately one-third of these patients remain unresponsive [4]. Therefore, it is essential for SOTRs to continue adhering to preventive measures even after vaccination and to consider options such as antibody prophylaxis [3]. Alternative therapeutic treatments should also be explored, especially for groups that may not be adequately protected by COVID-19 vaccination [5–7]. Monoclonal antibodies (mAbs) have shown promise in treating COVID-19 in SOTRs [8]. Tixagevimab/cilgavimab (Tix/Cil) is a mAb agent that neutralizes SARS-CoV-2 by binding to specific regions of the virus’s spike protein, preventing its attachment to the angiotensin-converting enzyme 2 receptor and subsequent infection [9]. This combination mAb agent is appropriate for early-stage COVID-19 in nonhospitalized individuals at risk of severe illness and is also effective for infection prevention and postexposure protection [10]. However, the U.S. Food and Drug Administration has withdrawn the emergency use authorization of Tix/Cil for treating high-risk patients with mild-to-moderate COVID-19 due to low efficacy against Omicron subvariants [11]. Real-world data indicate that Tix/Cil prophylaxis may effectively reduce mortality and hospitalization rates in SOTRs who become infected with the Omicron variant [9,12,13]. Two recent systematic reviews and meta-analyses have supported the effectiveness of preexposure prophylaxis with this drug combination in certain populations [14,15].
The protocol for this systematic review and meta-analysis was registered in the International Prospective Register of Systematic Reviews (PROSPERO) under the registration number CRD42024515919. The study adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, which are outlined in Supplementary Table 1 [19].
We systematically searched the PubMed, Cochrane Library, Embase, and Web of Science databases using keywords such as “SARS-CoV-2,” “COVID-19,” “solid organ transplant recipients,” “tixagevimab/cilgavimab,” and “Evusheld” up to December 15, 2023, to identify relevant studies. We also searched medRxiv and Google Scholar for additional records. Finally, we reviewed the reference lists of the selected studies. No language restrictions were applied. The specific search strategy for each database is detailed in Supplementary Table 2.
We included studies that met the following inclusion criteria: (1) population: SOTRs, (2) intervention: preexposure prophylaxis with Tix/Cil, (3) control: no Tix/Cil preexposure prophylaxis, and (4) reported outcomes of interest: mortality and hospitalization rates. We excluded studies that employed Tix/Cil as a treatment, studies detailing individuals who contracted COVID-19 prior to the study period, and articles structured as case reports, case series, or commentaries.
The risk of bias in the included studies was independently assessed by two researchers using the Risk of Bias in Non-randomized Studies of Interventions (ROBINS-I) tool [20]. This instrument provides a detailed evaluation of various potential biases, including those related to confounding, participant selection, classification of interventions, deviations from intended interventions, missing data, measurement of outcomes, and selection of reported results. To ensure a thorough assessment, the researchers applied a series of questions with five response options—yes, probably yes, no, probably no, or no information—to evaluate each domain. They then categorized the domain as low-risk, moderate-risk, serious-risk, critical-risk, or no information based on their findings. When the researchers’ assessments differed, they engaged in detailed discussions and consulted a third author to reconcile any discrepancies. Additionally, the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) tool was employed to rate the certainty of the evidence.
The data were independently collected by two researchers and included the following categories: (1) general study information, which encompassed the first author, year of publication, country of origin, and study design; (2) patient characteristics, detailing the sample size, sex distribution, and mean age of participants; (3) interventions, specifying the sample size, treatment dosage, and treatment duration; and (4) effectiveness outcomes, namely the incidence of SARS-CoV-2 infection, mortality rate, hospitalization rate, and intensive care unit (ICU) admission rate, with the last of these defined as admission to the ICU following a positive SARS-CoV-2 test result.
We used Comprehensive Meta-Analysis (ver. 3.0; Biostat) to assess the effectiveness of Tix/Cil as preexposure prophylaxis against COVID-19 in SOTRs. To evaluate the dichotomous data, we calculated odds ratios (ORs) with 95% confidence intervals (CIs). We considered heterogeneity to be present and significant if the I2 statistic exceeded 50% or if the P-value was less than 0.10. For studies with significant heterogeneity, we employed a random-effects model, with a fixed-effects model applied otherwise. Additionally, we conducted a sensitivity analysis, excluding studies that presented a high risk of bias.
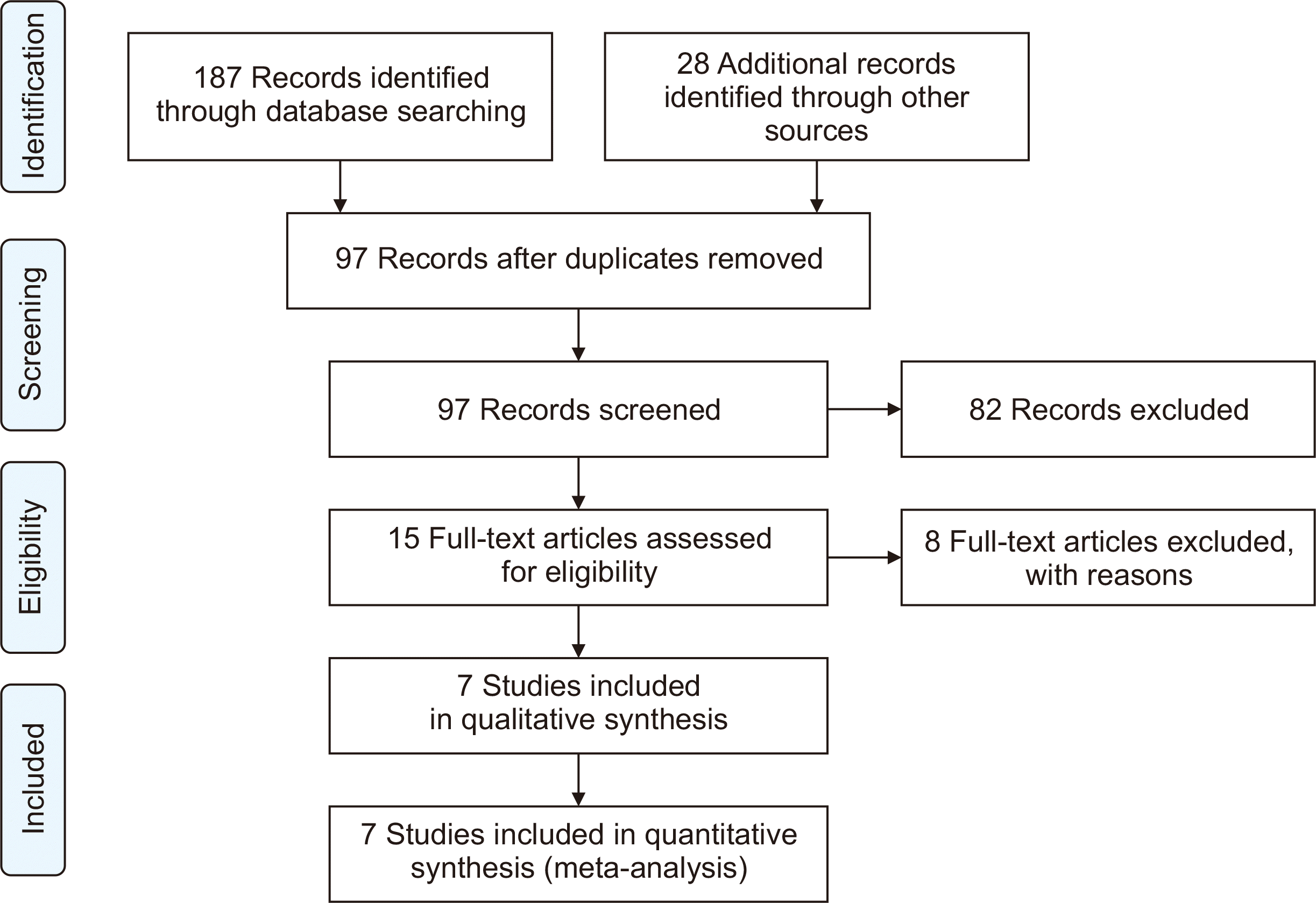
Fig. 1 presents a flow diagram detailing the selection process based on title, abstract, and full-text review. After the removal of duplicate records from an initial pool of 215, 15 studies were subjected to a full-text review to determine eligibility. Of these, eight studies were excluded for not meeting the inclusion criteria. Ultimately, seven retrospective studies [9,12,13,16–18,21], involving a total of 4,026 SOTRs, met the inclusion criteria and were examined in the meta-analysis. Most of the studies were conducted in the United States, with the kidney being the most commonly transplanted organ. Generally, SOTRs received Tix/Cil at a dosage of 150 mg/150 mg. However, one study [16] reported that 40.5% of participants received Tix/Cil at a dosage of 150 mg/150 mg, 59.0% at a dosage of 300 mg/300 mg, and 0.5% at a dosage of 450 mg/450 mg. The characteristics of the included studies are summarized in Table 1.
All studies exhibited a high risk of bias due to confounding and selection bias. The risk of bias associated with the classification of interventions, deviations from intended interventions, measurement of outcomes, and reporting of results was assessed as moderate. The risk of bias due to missing data was considered low. The results of the risk of bias assessment for each study are detailed in Supplementary Table 3. The certainty of evidence for all outcomes of interest is presented in Table 2.
SARS-CoV-2 infection
Across all seven studies [9,12,13,16–18,21] and a total of 3,538 SOTRs, the incidence of SARS-CoV-2 infection was compared between patients who received Tix/Cil preexposure prophylaxis and those who did not. Meta-analysis revealed a significant difference in the incidence of infection between these groups. Specifically, SOTRs who received Tix/Cil preexposure prophylaxis had a lower incidence of infection (OR, 0.30; 95% CI, 0.15–0.60; P=0.001; I2=87%) (Fig. 2).
All-cause mortality rate
In four studies [9,16–18], encompassing 1,766 SOTRs, mortality rates were reported for patients who received Tix/Cil preexposure prophylaxis and those who did not. The meta-analysis revealed no significant difference in mortality rate between these groups (OR, 0.25; 95% CI, 0.06–1.06; P=0.06; I2=0%) (Fig. 3).
Hospitalization rate
Hospitalization rates were analyzed based on 3,312 SOTRs across all seven studies [9,12,13,16–18,21]. The pooled analysis revealed a significant difference in hospitalization rate among SOTRs who received Tix/Cil preexposure prophylaxis relative to those who did not (OR, 0.24; 95% CI, 0.08–0.70; P=0.009; I2=67%) (Fig. 4).
Intensive care unit admission
Admission to the ICU was reported in two studies [17,18] involving 1,002 SOTRs who either did or did not receive Tix/Cil preexposure prophylaxis. Meta-analysis revealed significantly lower ICU admission rates among the patients who received prophylaxis relative to those who did not (OR, 0.07; 95% CI, 0.02–0.22; P<0.001, I2=0%) (Fig. 5).
Sensitivity analysis
After the exclusion of studies with a high risk of bias, sensitivity analysis indicated no significant change in the outcomes regarding the incidence of SARS-CoV-2 infection (OR, 0.34; 95% CI, 0.16–0.71; P=0.005; I2=88%) and hospitalization rate (OR, 0.26; 95% CI, 0.08–0.82; P=0.02; I2=72%).
While vaccination can augment the immunogenicity of COVID-19 vaccines in SOTRs, a considerable number of these patients do not develop a detectable humoral immune response, even after receiving a third dose [22]. Consequently, prophylactic interventions against SARS-CoV-2 are a high priority for SOTRs. The purpose of this study was to evaluate the effectiveness of Tix/Cil as preexposure prophylaxis in SOTRs, who face elevated risk of severe COVID-19. Seven studies were reviewed to assess the potential of Tix/Cil preexposure prophylaxis against SARS-CoV-2 in this population.
Our meta-analysis suggests that Tix/Cil preexposure prophylaxis may significantly reduce the incidence of SARS-CoV-2 infections in SOTRs. Preexposure prophylaxis has been established as an effective strategy for preventing SARS-CoV-2 infections among uninfected individuals at high risk [23]. This is particularly important for SOTRs, who exhibit a higher rate of COVID-19 breakthrough infections and worse outcomes after full or partial vaccination than individuals without immune dysfunction [24]. Our findings regarding the effectiveness of Tix/Cil preexposure prophylaxis are supported by recent systematic reviews and meta-analyses that investigated its role in reducing the rate of SARS-CoV-2 infections [14,15]. A meta-analysis by Soeroto et al. [15] demonstrated that Tix/Cil preexposure prophylaxis may significantly lower the incidence of SARS-CoV-2 infection in high-risk populations. A separate meta-analysis, conducted by Alhumaid et al. [14], reported similar results. mAbs have been shown to reduce the risk of SARS-CoV-2 infection among individuals at high risk of exposure to COVID-19 [9,13,25,26]. Studies have indicated that the administration of mAbs, such as bamlanivimab and the combination of casirivimab and imdevimab, can be effective in preventing SARS-CoV-2 infection in high-risk groups [25,26].
Our meta-analysis also revealed that preexposure prophylaxis with Tix/Cil in SOTRs demonstrated no significant clinical benefit in reducing COVID-19–related mortality. In contrast, two prior systematic reviews and meta-analyses have suggested that preexposure prophylaxis with this drug combination may significantly reduce COVID-19–related deaths in high-risk groups [14,15]. The discrepancy between these findings could stem from differences in the target populations. Our study exclusively included SOTRs, while the prior meta-analyses encompassed a broader set of populations. Despite pooled analysis of the included studies showing no significant benefit of Tix/Cil preexposure prophylaxis in reducing COVID-19–related deaths, three [9,12,16] of the four studies that detailed mortality rates in the present meta-analysis reported no deaths from SARS-CoV-2 infection in SOTRs who received prophylaxis. In contrast, COVID-19–related deaths were reported among SOTRs who did not receive the prophylactic combination. Several clinical trials and real-world studies have demonstrated the effectiveness of mAbs as both pre- and postexposure prophylaxis in reducing COVID-19–related deaths [9,18,25,26]. Therapeutically, a pooled analysis of retrospective studies indicated that treatment with mAbs may be associated with a decreased risk of death among SOTRs with COVID-19 [27].
Our meta-analysis findings indicate that SOTRs who received Tix/Cil preexposure prophylaxis had a significantly lower likelihood of hospitalization due to COVID-19 compared to those who did not receive prophylaxis. This outcome aligns with a systematic review and meta-analysis that examined the effectiveness of Tix/Cil as preexposure prophylaxis against COVID-19, which similarly demonstrated lower hospitalization rates among individuals receiving Tix/Cil [14]. Jurdi et al. [16] also found that the hospitalization rate from SARS-CoV-2 Omicron infection was lower in vaccinated SOTRs administered this drug combination as preexposure prophylaxis. Furthermore, our meta-analysis revealed that Tix/Cil preexposure prophylaxis against COVID-19 was effective in reducing ICU admissions in SOTRs. In line with our findings, a meta-analysis concluded that SOTRs who received the same combination to protect against COVID-19 were statistically less likely to be admitted to the ICU than those who did not [14].
Recent evidence has highlighted the resistance of certain SARS-CoV-2 strains to the combination of Tix/Cil [28,29]. Specifically, this combination has been found to be ineffective against Omicron sublineages including BA.1, BA.4, BA.5, and BA.2.75 [29]. However, research by Case et al. [28] indicates that Tix/Cil retains higher activity against the BA.2 and BA.5 sublineages. In contrast, Tix/Cil exhibits particularly low activity against BA.1. When comparing the efficacy across different sublineages, the activity of Tix/Cil is significantly better against BA.2 than BA.1, with its activity against BA.5 being intermediate but more similar to that against BA.2 [28].
Our study has several notable limitations. The retrospective studies included in the meta-analysis did not use propensity score matching to mitigate the risk of bias. Additionally, the number of studies available in the meta-analysis of certain outcomes, such as ICU admission, was limited. Furthermore, it was not feasible to conduct subgroup analyses by SARS-CoV-2 vaccination status and transplant type.
In conclusion, our meta-analysis indicates that preexposure prophylaxis with Tix/Cil for COVID-19 could be effective in decreasing the rates of SARS-CoV-2 infection, hospitalization, and ICU admission among SOTRs. However, it was not shown to be effective in reducing mortality rates within this population. These results may provide valuable insights for healthcare providers and researchers. Importantly, the evidence supporting these findings is of low to moderate quality. Additional real-world studies are necessary to verify the effectiveness of Tix/Cil preexposure prophylaxis against COVID-19 in SOTRs.
Supplementary materials can be found via https://doi.org/10.4285/ctr.24.0015.
REFERENCES
1. Clarke JA, Wiemken TL, Korenblat KM. 2022; Excess mortality among solid organ transplant recipients in the United States during the COVID-19 pandemic. Transplantation. 106:2399–407. DOI: 10.1097/TP.0000000000004341. PMID: 36042551. PMCID: PMC9696767.
2. Medina-Pestana J, Cristelli MP, Foresto RD, Tedesco-Silva H, Requião-Moura LR. 2022; The higher COVID-19 fatality rate among kidney transplant recipients calls for further action. Transplantation. 106:908–10. DOI: 10.1097/TP.0000000000004086. PMID: 35250005. PMCID: PMC9038247.

3. Peghin M, Graziano E, Grossi PA. 2022; SARS-CoV-2 vaccination in solid-organ transplant recipients. Vaccines (Basel). 10:1430. DOI: 10.3390/vaccines10091430. PMID: 36146506. PMCID: PMC9503203.

4. Yu B, Tamargo C, Brennan DC, Kant S. 2023; Measures to increase immunogenicity of SARS-CoV-2 vaccines in solid organ transplant recipients: a narrative review. Vaccines (Basel). 11:1755. DOI: 10.3390/vaccines11121755. PMID: 38140160. PMCID: PMC10748337.

5. Ryu TH, Kim HY, Ahn J, Oh JS, Kim JK. 2023; Delayed exacerbation of COVID-19 pneumonia in vaccinated kidney transplant recipients receiving immunosuppressants: a case series. Korean J Transplant. 37:63–8. DOI: 10.4285/kjt.22.0043. PMID: 37064773. PMCID: PMC10090830.

6. Amani B, Zareei S, Amani B, Zareei M, Zareei N, Shabestan R, et al. 2022; Artesunate, imatinib, and infliximab in COVID-19: a rapid review and meta-analysis of current evidence. Immun Inflamm Dis. 10:e628. DOI: 10.1002/iid3.628. PMID: 35634954. PMCID: PMC9092000.
7. Amani B, Akbarzadeh A, Amani B, Shabestan R, Khorramnia S, Navidi Z, et al. 2023; Comparative efficacy and safety of nirmatrelvir/ritonavir and molnupiravir for COVID-19: a systematic review and meta-analysis. J Med Virol. 95:e28889. DOI: 10.1002/jmv.28889. PMID: 37368841.
8. Yang M, Li T, Wang Y, Tran C, Zhao S, Ao G. 2022; Monoclonal antibody therapy improves severity and mortality of COVID-19 in organ transplant recipients: a meta-analysis. J Infect. 85:436–80. DOI: 10.1016/j.jinf.2022.06.027. PMCID: PMC9247112. PMID: 35788012.

9. Morado F, Davoudi R, Kawewat-Ho P, Nanda N, Cartus R, Shaikh SA. 2023; A single-center review of pre-exposure prophylaxis with tixagevimab-cilgavimab in solid organ transplant recipients. Transpl Infect Dis. 25:e14086. DOI: 10.1111/tid.14086. PMID: 37314092.
10. Capoluongo N, Mascolo A, Bernardi FF, Sarno M, Mattera V, di Flumeri G, et al. 2023; Retrospective analysis of a real-life use of tixagevimab-cilgavimab plus SARS-CoV-2 antivirals for treatment of COVID-19. Pharmaceuticals (Basel). 16:1493. DOI: 10.3390/ph16101493. PMID: 37895964. PMCID: PMC10609705.
11. Food and Drug Administration (FDA). 2023. FDA announces Evusheld is not currently authorized for emergency use in the U.S. [Internet]. FDA;Available from: https://www.fda.gov/drugs/drug-safety-and-availability/fda-announces-evusheld-not-currently-authorized-emergency-use-us. cited 2023 Dec 2.
12. Borštnar Š, Arnol M, Večerić Haler Ž, Mlinšek G. Preexposure prophylaxis against COVID-19 with tixagevimab/cilgavimab in Slovenian national cohort of kidney transplant recipients. Preprints.org [Preprint]. 2023. Available from: https://doi.org/10.20944/preprints202306.0122.v1. cited 2023 Oct 11. DOI: 10.20944/preprints202306.0122.v1.

13. Jordan SC, Joung SY, Wang M, Tran TA, Bravo M, Masoom H, et al. 2024; Assessing the post hoc effectiveness of tixagevimab-cilgavimab for prevention of SARS-CoV-2 infections in solid organ transplant recipients. Transpl Infect Dis. 26:e14182. DOI: 10.1111/tid.14182. PMID: 37885435. PMCID: PMC10922395.

14. Alhumaid S, Al Mutair A, Alali J, Al Dossary N, Albattat SH, Al HajjiMohammed SM, et al. 2022; Efficacy and safety of tixagevimab/cilgavimab to prevent COVID-19 (pre-exposure prophylaxis): a systematic review and meta-analysis. Diseases. 10:118. DOI: 10.3390/diseases10040118. PMID: 36547204. PMCID: PMC9777759.

15. Soeroto AY, Yanto TA, Kurniawan A, Hariyanto TI. 2023; Efficacy and safety of tixagevimab-cilgavimab as pre-exposure prophylaxis for COVID-19: a systematic review and meta-analysis. Rev Med Virol. 33:e2420. DOI: 10.1002/rmv.2420. PMID: 36617704.

16. Jurdi AA, Morena L, Cote M, Bethea E, Azzi J, Riella LV. 2022; Tixagevimab/cilgavimab pre-exposure prophylaxis is associated with lower breakthrough infection risk in vaccinated solid organ transplant recipients during the Omicron wave. Am J Transplant. 22:3130–6. DOI: 10.1111/ajt.17128. PMID: 35727916. PMCID: PMC9906353.

17. Bertrand D, Laurent C, Lemée V, Lebourg L, Hanoy M, Le Roy F, et al. 2022; Efficacy of anti-SARS-CoV-2 monoclonal antibody prophylaxis and vaccination on the Omicron variant of COVID-19 in kidney transplant recipients. Kidney Int. 102:440–2. DOI: 10.1016/j.kint.2022.05.007. PMID: 35618097. PMCID: PMC9125992.

18. Kaminski H, Gigan M, Vermorel A, Charrier M, Guirle L, Jambon F, et al. 2022; COVID-19 morbidity decreases with tixagevimab-cilgavimab preexposure prophylaxis in kidney transplant recipient nonresponders or low-vaccine responders. Kidney Int. 102:936–8. DOI: 10.1016/j.kint.2022.07.008. PMID: 35870641. PMCID: PMC9297679.

19. Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. 2015; Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 4:1. DOI: 10.1186/2046-4053-4-1. PMID: 25554246. PMCID: PMC4320440.

20. Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. 2016; ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 355:i4919. DOI: 10.1136/bmj.i4919. PMID: 27733354. PMCID: PMC5062054.

21. Grillini A, Stracener P, Scarola D, Lyons J, Dilling D. 2023; Use of tixagevimab and cilgavimab (Evusheld) and subsequent outcomes of SARS-CoV-2 infections in lung transplant recipients. J Heart Lung Transplant. 42(4 Suppl):S311. DOI: 10.1016/j.healun.2023.02.715. PMCID: PMC10068082.
22. Chen X, Luo D, Mei B, Du J, Liu X, Xie H, et al. 2023; Immunogenicity of COVID-19 vaccines in solid organ transplant recipients: a systematic review and meta-analysis. Clin Microbiol Infect. 29:441–56. DOI: 10.1016/j.cmi.2022.12.004. PMID: 36509376. PMCID: PMC9733302.

23. Ouyang J, Zaongo SD, Harypursat V, Li X, Routy JP, Chen Y. 2022; SARS-CoV-2 pre-exposure prophylaxis: a potential COVID-19 preventive strategy for high-risk populations, including healthcare workers, immunodeficient individuals, and poor vaccine responders. Front Public Health. 10:945448. DOI: 10.3389/fpubh.2022.945448. PMID: 36003629. PMCID: PMC9393547.

24. Sun J, Zheng Q, Madhira V, Olex AL, Anzalone AJ, Vinson A, et al. 2022; Association between immune dysfunction and COVID-19 breakthrough infection after SARS-CoV-2 vaccination in the US. JAMA Intern Med. 182:153–62. DOI: 10.1001/jamainternmed.2021.7024. PMID: 34962505. PMCID: PMC8715386.

25. O'Brien MP, Forleo-Neto E, Musser BJ, Isa F, Chan KC, Sarkar N, et al. 2021; Subcutaneous REGEN-COV antibody combination to prevent COVID-19. N Engl J Med. 385:1184–95. DOI: 10.1056/NEJMoa2109682. PMID: 34347950. PMCID: PMC8362593.
26. Cohen MS, Nirula A, Mulligan MJ, Novak RM, Marovich M, Yen C, et al. 2021; Effect of bamlanivimab vs placebo on incidence of COVID-19 among residents and staff of skilled nursing and assisted living facilities: a randomized clinical trial. JAMA. 326:46–55. DOI: 10.1001/jama.2021.8828. PMID: 34081073. PMCID: PMC8176388.

27. Amani B, Shabestan R, Rajabkhah K, Amani B. 2023; Sotrovimab in solid organ transplant recipients with COVID-19: a systematic review and meta-analysis. Korean J Transplant. 37:277–85. DOI: 10.4285/kjt.23.0038. PMID: 37916433. PMCID: PMC10772269.

28. Case JB, Mackin S, Errico JM, Chong Z, Madden EA, Whitener B, et al. 2022; Resilience of S309 and AZD7442 monoclonal antibody treatments against infection by SARS-CoV-2 Omicron lineage strains. Nat Commun. 13:3824. DOI: 10.1038/s41467-022-31615-7. PMID: 35780162. PMCID: PMC9250508.
29. Focosi D, Casadevall A. 2022; A critical analysis of the use of cilgavimab plus tixagevimab monoclonal antibody cocktail (EvusheldTM) for COVID-19 prophylaxis and treatment. Viruses. 14:1999. DOI: 10.3390/v14091999. PMID: 36146805. PMCID: PMC9505619.

Fig. 2
Forest plot of severe acute respiratory syndrome coronavirus 2 infection in the tixagevimab/cilgavimab (Tix/Cil) and control groups. CI, confidence interval.
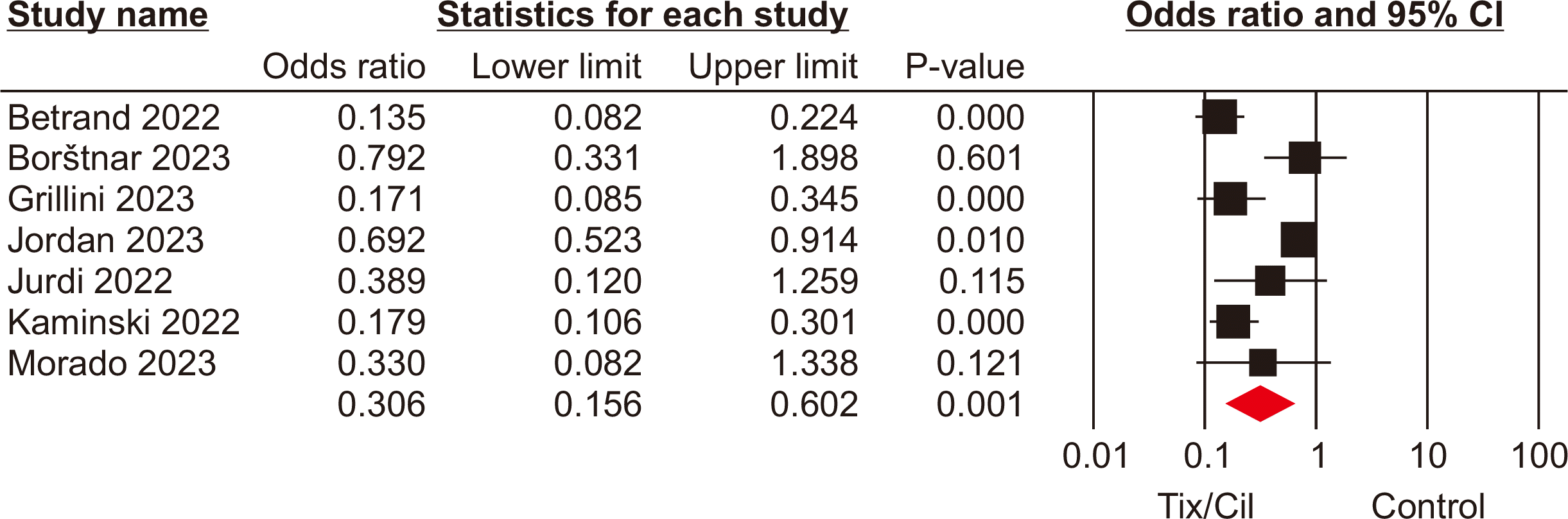
Fig. 3
Forest plot of mortality rate in the tixagevimab/cilgavimab (Tix/Cil) and control groups. CI, confidence interval.
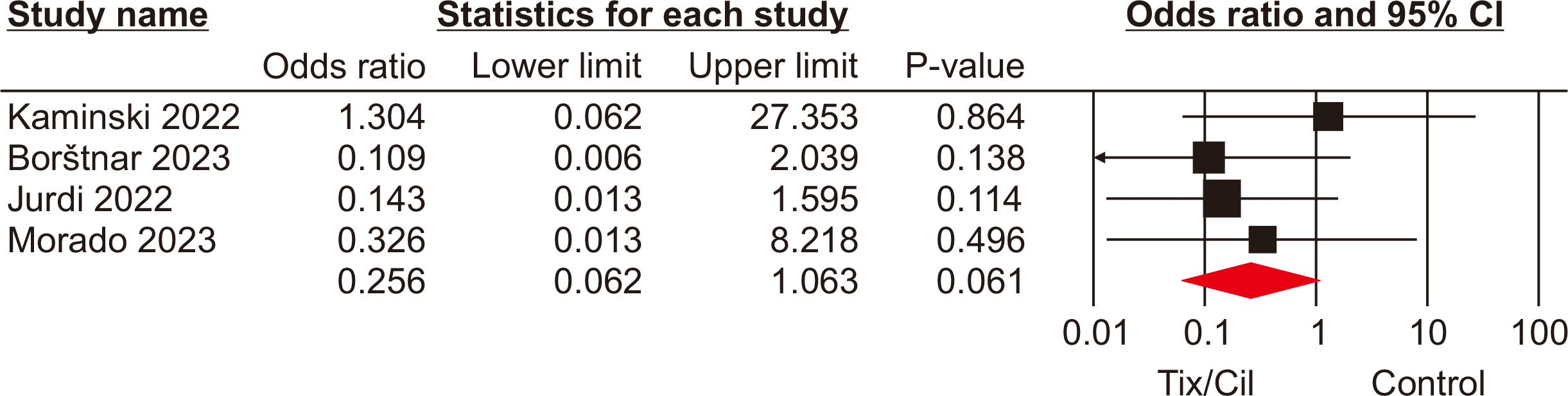
Fig. 4
Forest plot of hospitalization rate in the tixagevimab/cilgavimab (Tix/Cil) and control groups.
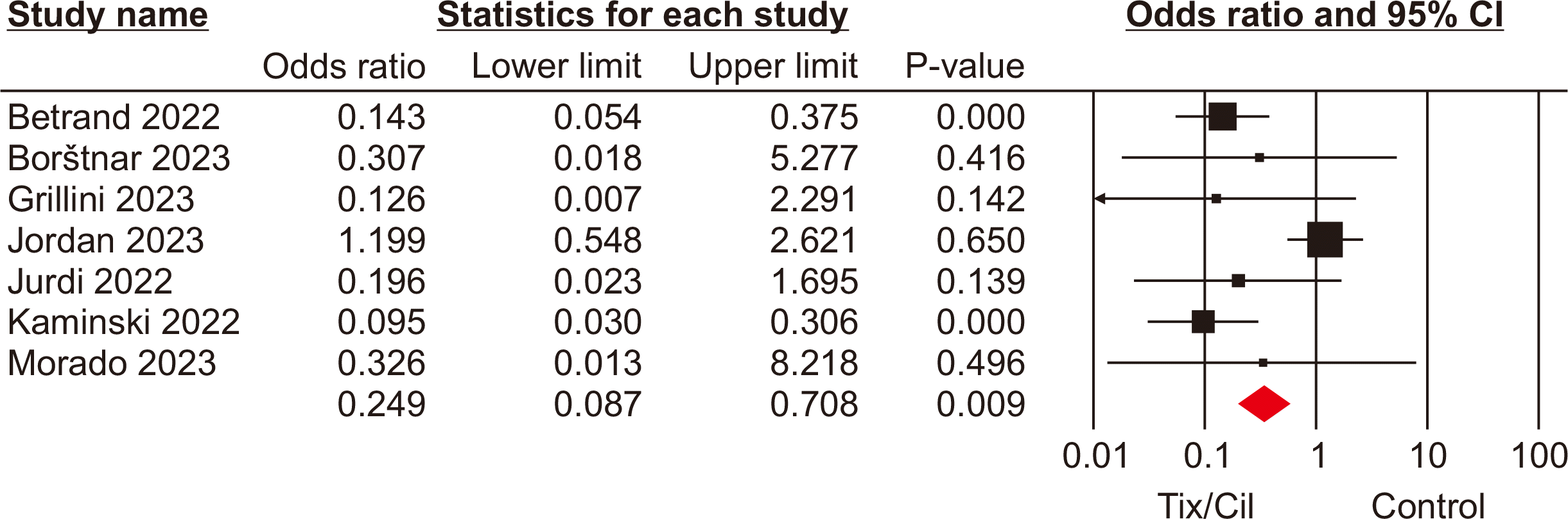
Fig. 5
Forest plot of intensive care unit admission rate in the tixagevimab/cilgavimab (Tix/Cil) and control groups.
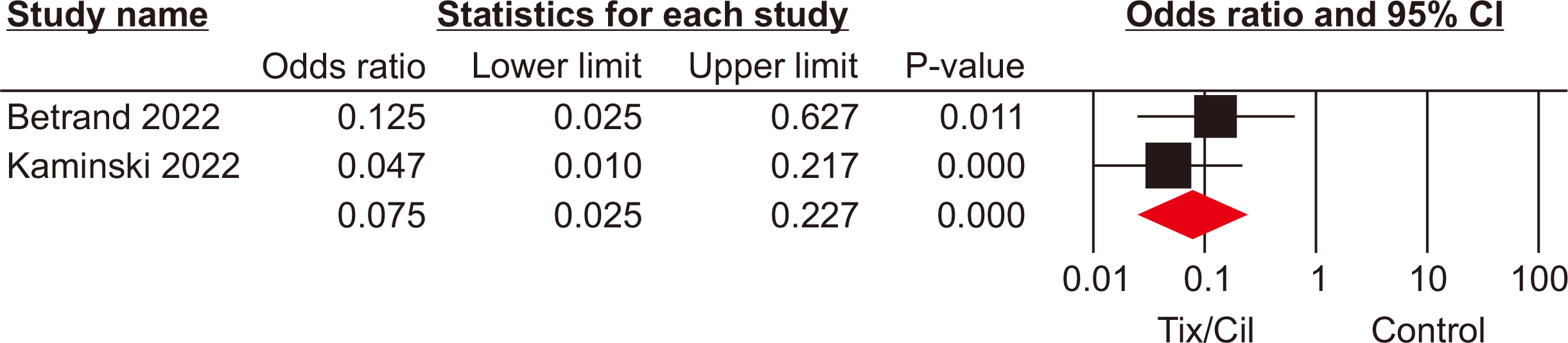
Table 1
Characteristics of studies included in the systematic review and meta-analysis
| Study | Country | Design | Transplant type | Sample size (n) | Tix/Cil | No Tix/Cil | COVID-19 vaccination rate (%) | Outcomes | ||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of patients | Mean age (yr) | No. of patients | Mean age (yr) | |||||||
| Bertrand et al. (2022) [17] | France | RS | Kidney | 860 | 412 | 60.2 | 288 | 56.3 | NA | Death, infection, hospitalization, ICU admission |
| Borštnar et al. (2023) [12] | Slovenia | RS | Kidney | 1,002 | 106 | 60 | 896 | 56 | >80 | Death, infection, hospitalization |
| Grillini et al. (2023) [21] | USA | RS | Lung | 289 | 136 | NA | 153 | NA | NA | Infection, hospitalization |
| Jordan et al. (2024) [13] | USA | RS | Kidney, heart, lung, liver, others | 911 | 381 | 60.53 | 530 | 54.51 | >96 | Infection, hospitalization |
| Jurdi et al. (2022) [16] | USA | RS | Kidney, liver, lung | 444 | 222 | NA | 222 | NA | NA | Death, infection, hospitalization |
| Kaminski et al. (2022) [18] | France | RS | Kidney | 430 | 333 | 60 | 97 | 58.3 | NA | Infection, hospitalization, ICU admission |
| Morado et al. (2023) [9] | USA | RS | Kidney, heart, lung, liver, others | 90 | 45 | 50.2 | 45 | 53.8 | 100 | Death, infection, hospitalization |
Table 2
Assessment of certainty of evidence using the GRADE approach for analyzed outcomes
| Evidence | Certainty assessment | Odds ratio (95% CI) | Certaintya) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No. of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other | |||
| SARS-CoV-2 infection | 7 | RS | Very serious | Not serious | Not serious | Very serious | None | 0.74 (0.12–4.53) | Low |
| Hospitalization rate | 7 | RS | Very serious | Not serious | Not serious | Serious | None | 0.78 (0.49–1.23) | Moderate |
| Mortality rate | 3 | RS | Very serious | Not serious | Not serious | Very serious | None | 0.75 (0.51–1.10) | Low |
| ICU admission | 2 | RS | Very serious | Not serious | Not serious | Very serious | None | 1.80 (0.24–13.40) | Low |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download